Accueil > Équipes scientifiques > Structure et dynamique des systèmes complexes isolés photoexcités (SYSIPHE) > CHIralité et spectroscoPIE (CHIPIE) > Spectrosccopy of cyclic dipeptides
Spectrosccopy of cyclic dipeptides
par - 3 août 2018 (modifié le 25 mars 2020)
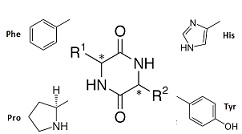
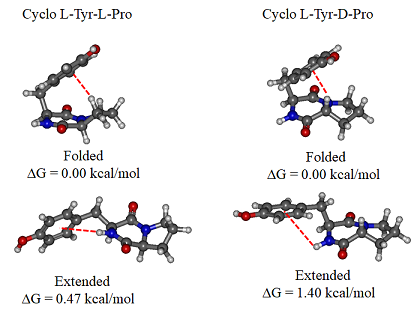
- Families of Tyr-Pro cyclo structures, LL on the left and LD on the right
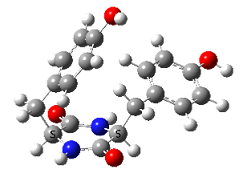
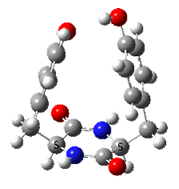
In contrast, very strong chirality dependence of the structure is observed for cyclo tyrosine-tyrosine, cyclo Tyr-Tyr. While both LL and LD diastereomers show the same “folded extended” structure as cyclo Phe-Phe, a structure involving a strong hydrogen bond between the hydroxyls of the two tyrosine residues is observed for LL only.
|
|
Publications
Conformational Study of the Jet-Cooled Diketopiperazine Peptide Cyclo Tyrosyl-Prolyl
A. Pérez-Mellor, I. Alata, V. Lepere, and A. Zehnacker
Journal of Physical Chemistry B, 2019, vol 123 (28), 6023-6033
Stereochemistry-dependent hydrogen bonds stabilise stacked conformations in jet-cooled cyclic dipeptides : (LD) vs. (LL) cyclo tyrosine–tyrosine
F. BenNasr, A. Pérez-Mellor, I. Alata, V. Lepere, N.-E. Jaïdane, and A. Zehnacker
Faraday Discussions, 2018, vol 212, 399-419
Chirality effects in the structures of jet-cooled bichromophoric dipeptides
A. Pérez-Mellor, I. Alata, V. Lepere, and A. Zehnacker
Journal of Molecular Spectroscopy, 2018, vol 349, 71-84
Vibrational circular dichroism of a 2,5‐diketopiperazine (DKP) peptide : Evidence for dimer formation in cyclo LL or LD diphenylalanine in the solid state
Ariel Pérez‐Mellor, Anne Zehnacker
Chirality, 2017, vol 29 (2), 89–96.
Dans la même rubrique :
- Philosophie de nos études
- Dichroïsme circulaire vibrationnel (VCD)
- Dichroïsme circulaire de photoélectron (PECD)
- Spectroscopie de dipeptides cycliques
- Le rôle des liaisons faibles
- Influence de la chiralité sur la structure de polyphénylalanines
- Reconnaissance moléculaire dans les dérivés de la quinine
- Rôle de l’énergie de déformation dans la formation des complexes
- Technique et développement expérimentaux
- Activités en posters
- Collaborations