Accueil > À la une > Understanding the coupling of the hydrated proton to its first solvation shell
Understanding the coupling of the hydrated proton to its first solvation shell
par - 23 octobre 2022
Despite its considerable importance, the "Grotthus" mechanism ; by which an ’excess’ proton or proton defect diffuses through the hydrogen bond network of water molecules in aqueous solution ; remains largely misunderstood. In liquid water, the solvation of these excess protons is represented in two main forms : H9O4+ (Eigen cation) and H5O2+ (Zundel cation). Very recently, experimental works concluded that the Zundel cation would be the predominant form, but even more recent simulations seem to indicate that it is, on the contrary, the Eigen cation which dominates.
Full-dimensional quantum simulations (up to 33 dimensions) based on the Multi-Layer Multi-Configuration Time-Dependent Hartree (ML-MCTDH) method have reproduced the Infra-red spectra of the Zundel and Eigen cations. These calculations have allowed a detailed understanding of the couplings between the proton and the water molecules that surround it in its first solvation shell.
The conclusion is that the H5O2+ subunit is the fundamental subunit that explains the two spectra. Embedded in the static environment of the parent Eigen cation, this subunit reproduces the positions and broadenings of its main excess-proton bands. In isolation, its spectrum reverts to the well-known Zundel ion. Hence, the dynamics of this subunit polarized by an environment suffice to explain the spectral signatures and anharmonic couplings of the solvated proton in its first solvation shell
The new results do not bring information about the relative populations of the two structures, but stress that the difficulty to understand if one structure dominates may partly come from the fact that the H5O2+ subunit can exhibit very similar spectral signatures compared to the Eigen cation when placed in a polarizing environment.
This finding, backed by the full quantum-mechanical approach, is suggestive of picturing the Eigen cation as three symmetric overlapping and strongly polarized Zundel H5O2+ subunits in the spirit of the classical ‘special pair dance’ models of the solvated proton.
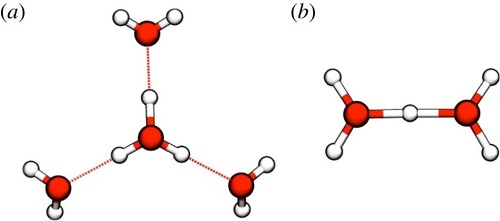
- Fig. 1
- Eigen cation, H9O4+ (a) ; Zundel cation, H5O2+ (b).
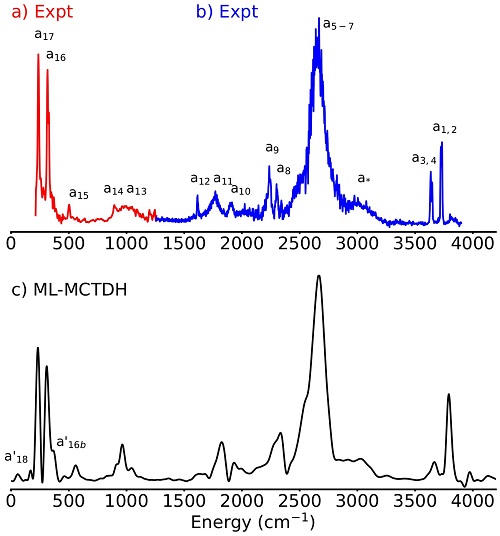
- Fig. 2
- Experimental and theoretical spectra of the Eigen cation
Contact : Fabien Gatti
Schröder, M., Gatti, F., Lauvergnat, D. et al. The coupling of the hydrated proton to its first solvation shell.
Nat Commun 13, 6170 (2022)
Dans la même rubrique :
- Caractériser les états excités d’atomes individuels grâce à la combinaison de la microscopie à effet tunnel et d’un laser impulsionnel
- MOOC de Paris Saclay : comprendre les nanosciences
- 3ème édition de la journée scientifique du FOrum de la Communauté Ultrarapide du plateau de Saclay
- Le télescope James Webb assiste en direct à la destruction d’un océan terrestre tous les mois
- Inauguration du laboratoire commun ISMO - Abbelight
- Jeudi de la recherche de Lou Barreau, jeudi 8 février à 18h
- L’art de mesurer la taille des grains interstellaires : ce que révèle JWST
- Chiralité induite sur une molécule achirale au synchrotron SOLEIL
- Webb cartographie la distribution de la glace dans un disque protoplanétaire.
- Workshop Nanoparticules Isolées 2024, 24 janvier de 9h30 à 14h
- Des valeurs plus précises des propriétés thermo-chimiques du CF, SiF et de leurs cations
- Production et manipulation d’un mélange de gaz quantiques dégénérés dans l’espace
- "Etonnante physique" vient de paraitre chez CNRS Editions
- Fête de la science à l’ISMO, dimanche 8 octobre
- Newsletter "3D Biomedical Imaging" de Laserlab forum
- Visite de 22 oeuvres d’art de plasticiens contemporains célèbres, exposées à l’ISMO
- Prix du meilleur poster de la journée CCPS à Ali Muhiedine
- Une nouvelle espèce carbonée cruciale détectée par le télescope spatial JWST dans un disque protoplanétaire
- Ecole thématique "Paris-Saclay Plasmonics School"
- Congrès de la SFP, 3-7 juillet 2023


