2024 |
Dupont, J., Hartwig, B., Le Barbu-Debus, K., Lepere, V., Guillot, R., Suhm, M. A., & Zehnacker, A. (2024). Homochiral vs. heterochiral preference in chiral self-recognition of cyclic diols. PCCP, 2622(1411), 1061011–1062111.

Résumé: The structure and clustering propensity of a chiral derivative of cis-1,2-cyclohexanediol, namely, 1-phenyl-cis-1,2-cyclohexanediol (cis-PCD), has been studied under supersonic expansion conditions by combining laser spectroscopy with quantum chemistry calculations. The presence of the phenyl substituent induces conformational locking relative to cis-1,2-cyclohexanediol (cis-CD), and only one conformer of the bare molecule is observed by both Raman and IR-UV double resonance spectroscopy. The homochiral preference inferred for the dimer formation at low enough temperature is in line with the formation of a conglomerate in the solid state. The change in clustering propensity in cis-PCD relative to trans-1,2-cyclohexanediol (trans-CD), which shows heterochiral preference, is explained by the presence of the phenyl substituent rather than the effect of cis-trans isomerism. Indeed the transiently chiral cis-CD also forms preferentially heterodimers, whose structure is very close to that of the corresponding trans-CD dimer.
|


|
2023 |
Imani, Z., Mundlapati, V. R., Brenner, V., Gloaguen, E., Le Barbu-Debus, K., Zehnacker, A., Robin, S., Aitken, D. J., & Mons, M. (2023). Non-covalent interactions reveal the protein chain δ conformation in a flexible single-residue model. ChemComm, 59, 1161–1164.
Résumé: The δ conformation is a local secondary structural feature in proteins that implicates a πamide N-H···N interaction between a backbone N atom and the NH of the following residue. Small molecule probes of this conformation have been limited so far to rigid proline-type models that may over-emphasize the significance of the interaction. We show here that, in thiacyclic amino acid derivatives with a sulphur atom in the γ-position, specific side-chain/backbone N-H···S interactions stabilize the δ conformation sufficiently to allow it to compete with classical C5 and C7 H-bonding conformers. With support from quantum chemistry, the δ-folded conformers have been characterized by IR spectroscopy in the gas phase. In solution, the IR absorption of the πamide N-H appears at 3450 cm-1, notably less red-shifted than in proline-type models, in a frequency range often considered as implicating a free NH motif and suggestive of very weak hydrogen bonding at best.
|


|
Nejad, A., Mellor, A. P. F., Lange, M., Alata, I., Zehnacker, A., & Suhm, M. A. (2023). Subtle hydrogen bond preference and dual Franck-Condon activity – the interesting pairing of 2-naphthol with anisole. PCCP, 25(15), 10427–10439.

Résumé: The hydrogen-bonded complexes between 2-naphthol (or beta-naphthol) and anisole are explored by detecting their IR absorption in the OH stretching range as well as their UV absorption by means of laser-induced fluorescence and resonance-enhanced two-photon UV ionisation. For the more stable cis and the metastable trans conformations of the OH group in 2-naphthol, hydrogen bonding to the oxygen atom of anisole is consistently detected in different supersonic jet expansions. Alternative hydrogen bonding to the aromatic ring of anisole remains elusive, although the majority of state-of-the-art hybrid DFT functionals with London dispersion correction and – less surprisingly – MP2 wavefunction theory predict it to be slightly more stable at zero-point level, unless three-body dispersion correction is added to the B3LYP-D3(BJ) approach. This changes at the CCSD(T) level, which forecasts an energy advantage of 1-3 kJ mol(-1) for the classical hydrogen bond arrangement even after including (DFT) zero-point energy contributions. The UV and IR spectra of the cis complex exhibit clear evidence for intensity redistribution of the primary OH stretch oscillator to combination states with the same low-frequency intermolecular bending mode by Franck-Condon-type vertical excitation mechanisms. This rare case of dual (vibronic and vibrational) Franck-Condon activity of a low-frequency mode invites future studies of homologues where aromatic ring docking of the OH group may be further stabilised, e.g. through anisole ring methylation.
|


|
Rouquet, E., Roy Chowdhury, M., Garcia, G. A., Nahon, L., Dupont, J., Lepere, V., Le Barbu-Debus, K., & Zehnacker, A. (2023). Induced photoelectron circular dichroism onto an achiral chromophore. Nat Commun, 1411(1), 629066.

Résumé: An achiral chromophore can acquire a chiral spectroscopic signature when interacting with a chiral environment. This so-called induced chirality is documented in electronic or vibrational circular dichroism, which arises from the coupling between electric and magnetic transition dipoles. Here, we demonstrate that a chiroptical response is also induced within the electric dipole approximation by observing the asymmetric scattering of a photoelectron ejected from an achiral chromophore in interaction with a chiral host. In a phenol-methyloxirane complex, removing an electron from an achiral aromatic pi orbital localised on the phenol moiety results in an intense and opposite photoelectron circular dichroism (PECD) for the two enantiomeric complexes with (R) and (S) methyloxirane, evidencing the long-range effect (~5 A) of the scattering chiral potential. This induced chirality has important structural and analytical implications, discussed here in the context of growing interest in laser-based PECD, for in situ, real time enantiomer determination.
|


|
2022 |
Dupont, J., Guillot, R., Lepère, V., & Zehnacker, A. (2022). Jet-cooled laser spectroscopy and solid-state vibrational circular dichroism of the cyclo-(Tyr-Phe) diketopiperazine dipeptide. Journal of Molecular Structure, 24, 19783–19791.
Résumé: The structures of the diastereomer diketopiperazine dipeptides cyclo(LTyr-LPhe) and cyclo-(DTyr-LPhe) are studied in the gas phase using resonance-enhanced two-photon ionization, IR/UV double resonance spectroscopy and quantum chemical calculation. Both diastereomers exhibit two conformations, with the tyrosine ring folded over the DKP ring and the phenylalanine ring extended outwards, or vice versa. The two diastereomers differ only slightly by the nature of weak CH···π and NH···π interactions. The crystal structure of cyclo(LTyr-LPhe) is determined by X-ray crystallography and is composed of monomers with folded tyrosine ring. The vibrational circular dichroism spectrum is interpreted by the existence of dimers in the solid state. Quantum chemical calculation shed light on the structural modifications between the gas phase and the solid state.
|


|
Fischer, T. L., Bödecker, M., Zehnacker-Rentien, A., Mata, R. A., & Suhm, M. A. (2022). Setting up the HyDRA blind challenge for the microhydration of organic molecules. PCCP, 24, 11442–11454.
Résumé: The procedure leading to the first HyDRA blind challenge for the prediction of water donor stretching vibrations in monohydrates of organic molecules is described. A training set of 10 monohydrates with experimentally known and published water donor vibrations is presented and a test set of 10 monohydrates with unknown or unpublished water donor vibrational wavenumbers is described together with relevant background literature. The rules for data submissions from computational chemistry groups are outlined and the planned publication procedure after the end of the blind challenge is discussed.
|


|
Jähnigen, S., Le Barbu-Debus, K., Guillot, R., Vuilleumier, R., & Zehnacker, A. (2022). How Crystal Symmetry Dictates Non-Local Vibrational Circular Dichroism in the Solid State. Angew. Chem. Int. Ed., 62(e20221559).
Résumé: Abstract Solid-State Vibrational Circular Dichroism (VCD) can be used to determine the absolute structure of chiral crystals, but its interpretation remains a challenge in modern spectroscopy. In this work, we investigate the effect of a twofold screw axis on the solid-state VCD spectrum in a combined experimental and theoretical analysis of P21 crystals of (S)-(+)-1-indanol. Even though the space group is achiral, a single proper symmetry operation has an important impact on the VCD spectrum, which reflects the supramolecular chirality of the crystal. Distinguishing between contributions originating from molecular chirality and from chiral crystal packing, we find that while IR absorption hardly depends on the symmetry of the space group, the situation is different for VCD, where completely new non-local patterns emerge. Understanding the two underlying mechanisms, namely gauge transport and direct coupling, will help to use VCD to distinguish polymorphic forms.
|


|
Le Barbu-Debus, K., Pérez-Mellor, A., Lepère, V., & Zehnacker, A. (2022). How change in chirality prevents β-amyloid type interaction in a protonated cyclic dipeptide dimer. PCCP, 24, 19783–19791.

Résumé: The protonated dimers of the diketopiperazine dipeptide cyclo (lPhe-lHis) and cyclo (lPhe-dHis) are studied by laser spectroscopy combined with mass spectrometry to shed light on the influence of stereochemistry on the clustering propensity of cyclic dipeptides. The marked spectroscopic differences experimentally observed in the hydride stretch region are well accounted for by the results of DFT calculations. Both diastereomeric protonated dimers involve a strong ionic hydrogen bond from the protonated imidazole ring of one monomer to the neutral imidazole nitrogen of the other. While this strong interaction is accompanied by a single NH⋯O hydrogen bond between the amide functions of the two moieties for the protonated dimer of cyclo (lPhe-dHis), that of cyclo (lPhe-lHis) involves two NH⋯O interactions, forming the motif of an antiparallel β sheet. Therefore, a change in chirality of the residue prevents the formation of the β sheet pattern observed in the amyloid type aggregation. These results emphasize the peculiar role of the histidine residue in peptide structure and interaction.
|


|
Perez-Mellor, A. F., Spezia, R., & Zehnacker, A. (2022). How Symmetry Influences the Dissociation of Protonated Cyclic Peptides. Symmetry, 14(4), 679.
Résumé: Protonated cyclic dipeptides undergo collision-induced dissociation, and this reaction mechanism strongly depends on the symmetry and the nature of the residues. We review the main dissociation mechanism for a series of cyclic dipeptides, obtained through chemical dynamics simulations. The systems range from the symmetrical cyclo-(glycyl-glycyl), with two possible symmetrical protonation sites located on the peptide ring, to cyclo-(tyrosyl-prolyl), where the symmetry of protonation sites on the peptide ring is broken by the dissimilar nature of the different residues. Finally, cyclo-(phenylalanyl-histidyl) shows a completely asymmetric situation, with the proton located on one of the dipeptide side chains, which explains the peculiar fragmentation mechanism induced by shuttling the proton, whose efficiency is strongly dependent on the relative chirality of the residues.
|


|
2021 |
Jahnigen, S., Zehnacker, A., & Vuilleumier, R. (2021). Computation of Solid-State Vibrational Circular Dichroism in the Periodic Gauge. J. Phys. Chem, 12(30), 7213–7220.
Résumé: We introduce a new theoretical formalism to compute solid-state vibrational circular dichroism (VCD) spectra from molecular dynamics simulations. Having solved the origin-dependence problem of the periodic magnetic gauge, we present IR and VCD spectra of (1S,2S)-trans-1,2-cyclohexanediol obtained from first-principles molecular dynamics calculations and nuclear velocity perturbation theory, along with the experimental results. Because the structure model imposes periodic boundary conditions, the common origin of the rotational strength has hitherto been ill-defined and was approximated by means of averaging multiple origins. The new formalism reconnects the periodic model with the finite physical system and restores gauge freedom. It nevertheless fully accounts for nonlocal spatial couplings from the gauge transport term. We show that even for small simulation cells the rich nature of solid-state VCD spectra found in experiments can be reproduced to a very satisfactory level.
|


|
Le Barbu-Debus, K., & Zehnacker, A. (2021). Competition between inter and intramolecular hydrogen bond evidenced by vibrational circular dichroism spectroscopy: The case of (1S,2R)-(−)-cis-1-amino-2-indanol. Chirality, 33(12), 858–874.
Résumé: Abstract The infrared (IR) absorption and vibrational circular dichroism (VCD) spectra of an intramolecularly hydrogen-bonded chiral amino-alcohol, (1S,2R)-(−)-cis-1-amino-2-indanol, are studied in DMSO-d6. The spectra are simulated at the density functional theory (DFT) level within the frame of the cluster-in-the-liquid model. Both IR and VCD spectra show a clear signature of the formation of intermolecular hydrogen bonds at the detriment of the intramolecular OH … N interaction present in the isolated molecule. Two solvent molecules are necessary to reproduce the experimental spectra. Whereas the first DMSO molecule captures the main spectral modifications due to hydrogen bond formation between the solute and the solvent, the second DMSO molecule is necessary for a good description of the Boltzmann contribution of the different complexes, based on their Gibbs free energy.
|


|
Mundlapati, V. R., Imani, Z., Goldsztejn, G., Gloaguen, E., Brenner, V., Le Barbu-Debus, K., Zehnacker-Rentien, A., Baltaze, J. - P., Robin, S., Mons, M., & Aitken, D. J. (2021). A theoretical and experimental case study of the hydrogen bonding predilection of S-methylcysteine. Amino Acids, 53(4), 621–633.

Résumé: S-containing amino acids can lead to two types of local NH···S interactions which bridge backbone NH sites to the side chain to form either intra- or inter-residue H-bonds. The present work reports on the conformational preferences of S-methyl-l-cysteine, Cys(Me), using a variety of investigating tools, ranging from quantum chemistry simulations, gas-phase UV and IR laser spectroscopy, and solution state IR and NMR spectroscopies, on model compounds comprising one or two Cys(Me) residues. We demonstrate that in gas phase and in low polarity solution, the C- and N-capped model compound for one Cys(Me) residue adopts a preferred C5–C6γ conformation which combines an intra-residue N–H···O=C backbone interaction (C5) and an inter-residue N–H···S interaction implicating the side-chain sulfur atom (C6γ). In contrast, the dominant conformation of the C- and N-capped model compound featuring two consecutive Cys(Me) residues is a regular type I β-turn. This structure is incompatible with concomitant C6γ interactions, which are no longer in evidence. Instead, C5γ interactions occur, that are fully consistent with the turn geometry and additionally stabilize the structure. Comparison with the thietane amino acid Attc, which exhibits a rigid cyclic side chain, pinpoints the significance of side chain flexibility for the specific conformational behavior of Cys(Me).
|


|
Pérez-Mellor, A., Alata, I., Lepere, V., Spezia, R., & Zehnacker-Rentien, A. (2021). Stereospecific collision-induced dissociation and vibrational spectroscopy of protonated cyclo (Tyr-Pro). International Journal of Mass Spectrometry, 465, 116590.

Résumé: The protonated cyclo (LTyr-LPro) and cyclo (LTyr-DPro) dipeptides based on a diketopiperazine (DKP) ring are studied by tandem mass spectrometry in a Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometer. Collision-induced dissociation (CID) and infrared multiple-photon dissociation (IRMPD) spectroscopy results are interpreted with the aid of quantum chemical calculations and chemical dynamics simulations. All the conformers identified for each diastereomer, denoted c-LLH+ and c-LDH+, respectively, are protonated on the carbonyl group of the tyrosine. The most stable form has an extended structure with the aromatic ring oriented outside the DKP ring; it is stabilized by an OH+…π interaction. Distinct IR signatures are obtained for the extended conformers of c-LLH+ and c-LDH+, which differ by the strength of the OH+…π interaction, much stronger in c-LLH+. Less stable species with the aromatic ring folded over the DKP ring are kinetically trapped in our experimental conditions, but their IR spectrum is identical for c-LLH+ and c-LDH+. The main collision-induced dissociation products of the protonated dipeptides are analyzed using chemical dynamics simulations. More efficient CID is observed for c-LDH+, in particular for the formation of the iminium ion of tyrosine. In contrast to the monomers, the protonated dimers of c-LLH+ and c-LDH+ show identical IR spectra. This is explained in terms of a structure involving a single strong OH+…O interaction between subunits not sensitive to the absolute configuration of the residues, i.e., from a folded protonated monomer to an extended neutral monomer.
|


|
Perez-Mellor, A., Le Barbu-Debus, K., Lepere, V., Alata, I., Spezia, R., & Zehnacker, A. (2021). Structure and collision-induced dissociation of the protonated cyclo His-Phe dipeptide: mechanistic studies and stereochemical effects. EUROPEAN PHYSICAL JOURNAL D, 75(6), 1–7.

Résumé: The role of stereochemical factors on the structure and the fragmentation paths of the protonated cyclic dipeptide cyclo histidine-phenylalanine is studied under ion traps conditions by combining tandem mass spectrometry, laser spectroscopy, quantum chemical calculations and chemical dynamics simulations. Vibrational spectroscopy obtained by Infrared Multiple Photon Dissociation (IRMPD) reveals a small difference between the two diastereomers, c-LLH+ and c-LDH+, arising mainly from ancillary CH center dot center dot center dot pi interactions. In contrast, there is a strong influence of the residues chirality on the collision-induced dissociation (CID) processes. Chemical dynamics simulations rationalize this effect and evidence that proton mobility takes place, allowing isomerization to intermediate cyclic structures that are different for c-LLH+ and c-LDH+, resulting in different barriers to proton mobility. This effect is related to the protonation of the imidazole ring. It contrasts with the minute stereochemical effects observed for other cyclic dipeptides in which the proton is borne by an amide CO.
|


|
2020 |
Hartwig, B., Lange, M., Poblotzki, A., Medel, R., Zehnacker, A., & Suhm, M. A. (2020). The reduced cohesion of homoconfigurational 1,2-diols. PCCP, 22(3), 1122–1136.

Résumé: By a combination of linear FTIR and Raman jet spectroscopy, racemic trans-1,2-cyclohexanediol is shown to form an energetically unrivalled S4-symmetric heterochiral dimer in close analogy to 1,2-ethanediol. Analogous experiments with enantiopure trans-1,2-cyclohexanediol reveal the spectral signature of at least three unsymmetric homochiral dimers. A comparison to signal-enhanced spectra of 1,2-ethanediol and to calculations uncovers at least three transiently homochiral dimer contributions as well. In few of these dimer structures, the intramolecular OH⋯O contact present in monomeric 1,2-diols survives, despite the kinetic control in supersonic jet expansions. This provides further insights into the dimerisation mechanism of conformationally semi-flexible molecules in supersonic jets. Racemisation upon dimerisation is shown to be largely quenched under jet cooling conditions, whereas it should be strongly energy-driven at higher temperatures. The pronounced energetic preference for heterochiral aggregation of vicinal diols is also discussed in the context of chirality-induced spin selectivity.
|


|
Hirata, K., Mori, Y., Ishiuchi, S. -ichi, Fujii, M., & Zehnacker, A. (2020). Chiral discrimination between tyrosine and β-cyclodextrin revealed by cryogenic ion trap infrared spectroscopy. PCCP, 22(43), 24887–24894.

Résumé: Complexes of permethylated β-cyclodextrin (β-MCD) with the two enantiomers of protonated tyrosine (l- and d-TyrH+) are studied by cryogenic ion trap infrared photo-dissociation spectroscopy. The vibrational spectra in the OH/NH stretch and fingerprint regions are assigned based on density functional theory calculations. The spectrum of both l- and d-TyrH+ complexes contains features characteristic of a first structure with ammonium and acid groups of the amino acid simultaneously interacting with the β-MCD, the phenolic OH remaining free. A second structure involving additional interaction between the phenolic OH and the β-MCD is observed only for the complex with d-TyrH+. The larger abundance of the d-TyrH+ complex in the mass spectrum is tentatively explained in terms of (1) better insertion of d-TyrH+ within the cavity with the hydrophobic aromatic moiety less exposed to hydrophilic solvent molecules and (2) a stiff structure involving three interaction points, namely the ammonium, the phenolic OH and the carboxylic acid OH, which is not possible for the complex with l-TyrH+. The recognition process does not occur through size effects that induce complementarity to the host molecule but specific interactions. These results provide a comprehensive understanding of how the cyclodextrin recognises a chiral biomolecule.
|


|
Imani, Z., Mundlapati, V. R., Goldsztejn, G., Brenner, V., Gloaguen, E., Guillot, R., Baltaze, J. P., Le Barbu-Debus, K., Robin, S., Zehnacker, A., Mons, M., & Aitken, D. J. (2020). Conformation control through concurrent N–H⋯S and N–H⋯O=C hydrogen bonding and hyperconjugation effects. CHEMICAL SCIENCE, 11(34), 9191–9197.

Résumé: In addition to the classical N-HMIDLINE HORIZONTAL ELLIPSISO-C non-covalent interaction, less conventional types of hydrogen bonding, such as N-HMIDLINE HORIZONTAL ELLIPSISS, may play a key role in determining the molecular structure. In this work, using theoretical calculations in combination with spectroscopic analysis in both gas phase and solution phase, we demonstrate that both these H-bonding modes exist simultaneously in low-energy conformers of capped derivatives of Attc, a thietane alpha-amino acid. 6-Membered ring inter-residue N-HMIDLINE HORIZONTAL ELLIPSISS interactions (C6(gamma)), assisted by hyperconjugation between the thietane ring and the backbone, combine with 5-membered ring intra-residue backbone N-HMIDLINE HORIZONTAL ELLIPSISO-C interactions (C5) to provide a C5-C6(gamma)feature that stabilizes a planar geometry in the monomer unit. Two contiguous C5-C6(gamma)features in the planar dimer implicate an unprecedented three-centre H-bond of the type C-OMIDLINE HORIZONTAL ELLIPSISH(N)MIDLINE HORIZONTAL ELLIPSISSR2, while the trimer adopts two C5-C6(gamma)features separated by a Ramachandran alpha-type backbone configuration. These low-energy conformers are fully characterized in the gas phase and support is presented for their existence in solution state.
|


|
Le Barbu-Debus, K., Bowles, J., Jähnigen, S., Clavaguéra, C., Calvo, F., Vuilleumier, R., & Zehnacker, A. (2020). Assessing cluster models of solvation for the description of vibrational circular dichroism spectra: synergy between static and dynamic approaches. PCCP, 22, 26047–26068.

Résumé: Solvation effects are essential for defining the shape of vibrational circular dichroism (VCD) spectra. Several approaches have been proposed to include them into computational models for calculating VCD signals, in particular those resting on the “cluster-in-a-liquid” model. Here we examine the capabilities of this ansatz on the example of flexible (1S,2S)-trans-1-amino-2-indanol solvated in dimethyl sulfoxide (DMSO). We compare cluster sets obtained from static calculations with results from explicit molecular dynamics (MD) trajectories based on either force field (FF) or first-principles (FP) methods. While the FFMD approach provides a broader sampling of configurational space, FPMD and time-correlation functions of dipole moments account for anharmonicity and entropy effects in the VCD calculation. They provide a means to evaluate the immediate effect of the solvent on the spectrum. This survey singles out several challenges associated with the use of clusters to describe solvation effects in systems showing shallow potential energy surfaces and non-covalent interactions. Static structures of clusters involving a limited number of solvent molecules satisfactorily capture the main effects of solvation in the bulk limit on the VCD spectra, if these structures are correctly weighted. The importance of taking into consideration their fluxionality, i.e. different solvent conformations sharing a same hydrogen bond pattern, and the limitations of small clusters for describing the solvent dynamics are discussed.
|


|
|
Le Barbu-Debus, K., Scuderi, D., Lepère, V., & Zehnacker, A. (2020). Homochiral vs. heterochiral sodium core dimers of tartaric acid esters: A mass spectrometry and vibrational spectroscopy study. Journal of Molecular Structure, 1205, 127583.
|


|
Li, X., Porcino, M., Martineau-Corcos, C., Guo, T., Xiong, T., Zhu, W. F., Patriarche, G., Pechoux, C., Perronne, B., Hassan, A., Kummerle, R., Michelet, A., Zehnacker-Rentien, A., Zhang, J. W., & Gref, R. (2020). Efficient incorporation and protection of lansoprazole in cyclodextrin metal-organic frameworks. International Journal of Pharmaceutics, 585, 119442.

Résumé: Lansoprazole (LPZ) is an acid pump inhibitor, which readily degrades upon acidic or basic conditions and under heating. We investigated here LPZ stability upon incorporation in particles made of cyclodextrin metal-organic frameworks (CD-MOFs). LPZ loaded CD-MOFs were successfully synthesized, reaching high LPZ payloads of 23.2 +/- 2.1 wt%, which correspond to a molar ratio of 1:1 between LPZ and gamma-CD. The homogeneity of LPZ loaded CD-MOFs in terms of component distribution was confirmed by elemental mapping by STEM-EDX. Both CTAB, the surfactant used in the CD-MOFs synthesis, and LPZ compete for their inclusion in the CD cavities. CTAB allowed obtaining regular cubic particles of around 5 μm with 15 wt% residual CTAB amounts. When LPZ was incorporated, the residual CTAB amount was less than 0.1 wt%, suggesting a higher affinity of LPZ for the CDs than CTAB. These findings were confirmed by molecular simulations. Vibrational circular dichroism studies confirmed the LPZ incorporation inside the CDs. Solid-state NMR showed that LPZ was located in the CDs and that it remained intact even after three years storage. Remarkably, the CD-MOFs matrix protected the drug upon thermal decomposition. This study highlights the interest of CD-MOFs for the incorporation and protection of LPZ.
|


|
Pérez-Mellor, A., Le Barbu-Debus, K., & Zehnacker, A. (2020). Solid-state synthesis of cyclo LD-diphenylalanine: A chiral phase built from achiral subunits. Chirality, 32(5), 693–703.

Résumé: Abstract The solid-state structure of LL/DD or LD/DL diphenylalanine diluted in KBr pellets is studied by infrared (IR) absorption and vibrational circular dichroism (VCD) spectroscopy. The structure depends on the absolute configuration of the residues. The natural LL diphenylalanine exists as a mixture of neutral and zwitterionic structures, depending on the humidity of the sample, while mostly the zwitterion is observed for LD diphenylalanine whatever the experimental conditions. The system undergoes spontaneous cyclization upon heating at 125°C, resulting to the formation of a diketopiperazine (DKP) dipeptide as the only product. The reaction is faster for LD than for LL diphenylalanine. As expected, LL and DD diphenylalanine react to form the LL and DD enantiomers of cyclo diphenylalanine. Interestingly, the DKP dipeptides formed from the LD or DL diphenylalanine show unexpected optical activity, with opposite VCD spectra for the products formed from the LD and DL reagents. This is explained in terms of chirality synchronization between the monomers within the crystal, which retain the symmetry of the reagent, resulting to the formation of a new chiral phase made from transiently chiral molecules.
|


|
2019 |
Ben Nasr, F., Alata, I., Scuderi, D., Lepere, V., Brenner, V., Jaidane, N. E., & Zehnacker, A. (2019). Effects of complexation with sulfuric acid on the photodissociation of protonated Cinchona alkaloids in the gas phase. Physical Chemistry Chemical Physics, 21(28), 15439–15451.

Résumé: The effect of complexation with sulfuric acid on the photo-dissociation of protonated Cinchona alkaloids, namely cinchonidine (Cd), quinine (Qn) and quinidine (Qd), is studied by combining laser spectroscopy with quantum chemical calculations. The protonated complexes are structurally characterized in a room-temperature ion trap by means of infra-red multiple photon dissociation (IRMPD) spectroscopy in the fingerprint and the nu(XH) (X = C, N, O) stretch regions. Comparison with density functional theory calculations including dispersion (DFT-D) unambiguously shows that the complex consists of a doubly protonated Cinchona alkaloid strongly bound to a bisulfate HSO4- anion, which bridges the two protonated sites of the Cinchona alkaloid. UV excitation of the complex does not induce loss of specific photo fragments, in contrast to the protonated monomer or dimer, for which photo-specific fragments were observed. Indeed the UV-induced fragmentation pattern is identical to that observed in collision-induced dissociation experiments. Analysis of the nature of the first electronic transitions at the second order approximate coupled-cluster level (CC2) explains the difference in the behavior of the complex relative to the monomer or dimer towards UV excitation.
|


|
Declerck, V., Perez-Mellor, A., Guillot, R., Aitken, D. J., Mons, M., & Zehnacker, A. (2019). Vibrational circular dichroism as a probe of solid-state organisation of derivatives of cyclic beta-amino acids: Cis- and trans-2-aminocyclobutane-1-carboxylic acid. Chirality, 31(8), 547–560.

Résumé: Peptide models built from cis- and trans-2-aminocyclobutane-1-carboxylic acids (ACBCs) are studied in the solid phase by combining Fourier-transform infrared spectroscopy (FTIR) absorption spectroscopy, vibrational circular dichroism (VCD), and quantum chemical calculations using density functional theory (DFT). The studied systems are N-tert-butyloxycarbonyl (Boc) derivatives of 2-aminocyclobutanecarboxylic acid (ACBC) benzylamides, namely Boc-(cis-ACBC)-NH-Bn and Boc-(trans-ACBC)-NH-Bn. These two diastereomers show very different VCD signatures and intensities, which of the trans-ACBC derivative being one order of magnitude larger in the region of the nu (CO) stretch. The spectral signature of the cis-ACBC derivative is satisfactorily reproduced by that of the monomer extracted from the solid-state geometry of related ACBC derivatives, which shows that no long-range effects are implicated for this system. In terms of hydrogen bonds, the geometry of this monomer is intermediate between the C6 and C8 structures (exhibiting a 6- or 8-membered cyclic NHMIDLINE HORIZONTAL ELLIPSISO hydrogen bond) previously evidenced in the gas phase. The benzyl group must be in an extended geometry to reproduce satisfactorily the shape of the VCD spectrum in the nu (CO) range, which qualifies VCD as a potential probe of dispersion interaction. In contrast, reproducing the IR and VCD spectrum of the trans-ACBC derivative requires clusters larger than four units, exhibiting strong intermolecular H-bonding patterns. A qualitative agreement is obtained for a tetramer, although the intensity enhancement is not reproduced. These results underline the sensitivity of VCD to the long-range organisation in the crystal.
|


|
|
Pérez-Mellor A. Zehnacker A. (2019). Chirality Effects in Jet-Cooled Cyclic Dipeptides. In Ebata T. Fujii M. (Ed.), Physical Chemistry of Cold Gas-Phase Functional Molecules and Clusters (pp. 63–87). Singapore: Springer.
|


|
Perez-Mellor, A., Alata, I., Lepere, V., & Zehnacker, A. (2019). Conformational Study of the Jet-Cooled Diketopiperazine Peptide Cyclo Tyrosyl-Prolyl. Journal Of Physical Chemistry B, 123(28).

Résumé: The conformational landscape of the diketopiperazine (DKP) dipeptide built on tyrosine and proline, namely, cyclo Tyr-Pro, is studied by combining resonance-enhanced multiphoton ionization, double resonance infrared ultraviolet (IR-UV) spectroscopy, and quantum chemical calculations. Despite the geometrical constraints due the two aliphatic rings, DKP and proline, cyclo Tyr-Pro is a flexible molecule. For both diastereoisomers, cyclo LTyr-LPro and cyclo LTyr-DPro, two structural families coexist under supersonic jet conditions. In the most stable conformation, the aromatic tyrosine substituent is folded over the DKP ring (g(+) geometry of the aromatic ring) as it is in the solid state. The other structure is completely extended (g(-) geometry of the aromatic ring) and resembles that proposed for the vapor phase. IR-UV results are not sufficient for unambiguous assignment of the observed spectra to either folded or extended conformations and the simulation of the vibronic pattern of the S-0-S-1 transition is necessary. Still, the comparison between IR-UV results and anharmonic calculations allows explanation of the minor structural differences between cyclo LTyr-LPro and cyclo LTyr-DPro in terms of different NH center dot center dot center dot pi and CH center dot center dot center dot pi interactions.
|


|
Tamura, M., Sekiguchi, T., Ishiuchi, S., Zehnacker-Rentien, A., & Fujii, M. (2019). Can the Partial Peptide SIVSF of the beta(2)-Adrenergic Receptor Recognize Chirality of the Epinephrine Neurotransmitter? Journal Of Physical Chemistry Letters, 10(10), 2470–2474.

Résumé: Chirality plays an essential role in biological molecular recognition, such as neurotransmission. Here, we applied electrospray-cold ion trap spectroscopy to complexes of a partial binding motif SIVSF of a beta(2)-adrenergic receptor pocket with L- and D-epinephrine AdH(+). The ultraviolet spectrum of the SIVSF-AdH(+) complex changed drastically when L-AdH(+) was replaced by its enantiomer. The isomer-selected infrared spectra revealed that D-AdH(+) was bound to SIVSF by its protonated amino-group or a single catechol OH and induced nonhelical secondary structures of SIVSF. This is in sharp contrast to the helical SIVSF complex with L-AdH(+), which is close to the natural binding structure with two catechol OHs binding in the receptor. This shows that a short pentapeptide SIVSF can distinguish the chirality of the ligand AdH(+) as well as the receptor. This stereoselectivity is suggested to arise from an additional interaction involving the hydroxyl group on the chiral carbon.
|


|
2018 |
|
Babikov, D., Benoit, D., Bowman, J., Burd, T., Clary, D., Donovan, R., Fischer, I., Gianturco, F., Hochlaf, M., Kar, S., Kirrander, A., Leone, S., Malcomson, T., Manthe, U., McCoy, A. B., Petersen, J., Richardson, J., Slavicek, P., Stoecklin, T., Szalewicz, K., van der Avoird, A., Wester, R., Worth, G., & Zehnacker-Rentien, A. (2018). Quantum dynamics of isolated molecules: general discussion. Faraday Discussions, 212, 281–306.
|


|
|
Bacic, Z., Benoit, D., Besemer, M., Bowman, J., Bradforth, S., Clary, D., Donovan, R., Fischer, I., Gianturco, F., Hochlaf, M., Houston, P., Knowles, P., Leone, S., Linguerri, R., Manthe, U., McCoy, A. B., Petersen, J., Richardson, J., Shan, X., Slavicek, P., Stoecklin, T., Szalewicz, K., van der Avoird, A., Wester, R., Worth, G., & Zehnacker-Rentien, A. (2018). Precise characterisation of isolated molecules: general discussion. Faraday Discussions, 212, 137–155.
|


|
|
Bacic, Z., Benoit, D., Biczysko, M., Bowman, J., Bradforth, S., Burd, T., Chambaud, G., Clary, D., Crepin, C., Dracinsky, M., Felker, P., Fischer, I., Gianturco, F., Hochlaf, M., Kouril, K., Kratochvilova, I., Liu, C. M., McCoy, A., Miyazaki, J., Mouhib, H., Richardson, J., Slavicek, P., Stoecklin, T., Szalewicz, K., van der Avoird, A., & Zehnacker-Rentien, A. (2018). Molecules in confinement in clusters, quantum solvents and matrices: general discussion. Faraday Discussions, 212, 569–601.
|


|
|
Ban, L., Bowman, J., Bradforth, S., Chambaud, G., Dracinsky, M., Fischer, I., Gora, R., Hochlaf, M., Janicki, M., Kirrander, A., McCoy, A. B., Petersen, J., Richardson, J., Slavicek, P., Szalewicz, K., & Zehnacker-Rentien, A. (2018). Molecules in confinement in liquid solvents: general discussion. Faraday Discussions, 212, 383–397.
|


|
BenNasr, F., Perez-Mellor, A., Alata, I., Lepere, V., Jaidane, N. E., & Zehnacker, A. (2018). Stereochemistry-dependent hydrogen bonds stabilise stacked conformations in jet-cooled cyclic dipeptides: (LD) vs. (LL) cyclo tyrosine-tyrosine. Faraday Discussions, 212, 399–419.

Résumé: Tyrosine-containing cyclic dipeptides based on a diketopiperazine (DKP) ring are studied under jet-cooled conditions using resonance-enhanced multi-photon ionisation (REMPI), conformer-selective IR-UV double resonance vibrational spectroscopy and quantum chemical calculations. The conformational landscape of the dipeptide containing natural L tyrosine (Tyr), namely c-LTyr-LTyr strongly differs from that of its diastereomer c-LTyr-DTyr. A similar family of conformers exists in both systems, with one aromatic ring folded on the dipeptide DKP ring and the other one extended. Weak NH and CH interactions are observed, which are slightly different in c-LTyr-LTyr and c-LTyr-DTyr. These structures are identical to those of LL and LD cyclo diphenylalanine, which only differ from c-Tyr-Tyr by the absence of hydroxyl on the benzene rings. While this is the only conformation observed for c-LTyr-DTyr, c-LTyr-LTyr exhibits an additional form stabilised by the interaction of the two hydroxyls, in which the two aromatic rings are in a stacked geometry. Stereochemical effects are still visible in the radical cation, for which one structure is observed for c-LTyr-DTyr, while the spectrum of the c-LTyr-LTyr radical cation is explained in terms of two co-existing structures.
|


|
Bouchet, A., Klyne, J., Ishiuchi, S. - I., Dopfer, O., Fujii, M., & Zehnacker, A. (2018). Stereochemistry-dependent structure of hydrogen-bonded protonated dimers: the case of 1-amino-2-indanol. Phys Chem Chem Phys, 20, 12430–12443.

Résumé: To understand the role of chirality in shaping biological supramolecular systems it is instructive to visualize the subtle effects of stereochemistry on the structure of model aggregates at the molecular level. Here, we apply conformer-specific IR-UV double-resonance laser spectroscopy in a cold ion trap to derive a detailed description of the protonated homodimers of (1R,2S)-cis- and (1R,2R)-trans-1-amino-2-indanol (c-AI2H+, t-AI2H+). Although the protonated monomers (c-AIH+, t-AIH+) only differ by the chirality of one carbon atom, their conformations are clearly distinct. c-AIH+ has an intramolecular NH+O hydrogen bond (H-bond), while t-AIH+ lacks such an interaction. This has crucial consequences on the geometry and stability of the corresponding c-AI2H+ and t-AI2H+ dimers. While there is a competition between intra- and intermolecular H-bonds in c-AI2H+, the formation of t-AI2H+ does not require deformation of the monomers. This difference results in higher binding energies of t-AI2H+ compared to c-AI2H+. To optimize the H-bond network, the two dimers do not necessarily involve the corresponding most stable monomers. c-AI2H+ and t-AI2H+ differ in their UV photodissociation mass spectra and in their electronic spectra, which suggests different geometries also in the excited state.
|


|
Le Barbu-Debus, K., Scherrer, A., Bouchet, A., Sebastiani, D., Vuilleumier, R., & Zehnacker, A. (2018). Effect of puckering motion and hydrogen bond formation on the vibrational circular dichroism spectrum of a flexible molecule: the case of (S)-1-indanol. Phys Chem Chem Phys, 20(21), 14635–14646.

Résumé: The influence of flexibility and hydrogen bond formation on the IR absorption and vibrational circular dichroism (VCD) spectrum of a floppy protic molecule, namely, (S)-1-indanol, is studied in both non-polar CCl4 and polar DMSO solvents. The experimental IR absorption and VCD spectra obtained by Fourier transform spectroscopy are interpreted using both static density functional theory (DFT) calculations and first principles molecular dynamics (FPMD) within DFT, using the nuclear velocity perturbation theory (NVPT). Simulation of the spectra based on static optimised geometries is not sufficient in CCl4 and going beyond static calculations is mandatory for satisfactorily reproducing the VCD spectra. The FPMD results obtained in DMSO indicate that (S)-1-indanol is hydrogen-bonded to one DMSO molecule. As a result, static “cluster-in-the-bulk” DFT calculations in which the solute-solvent interaction is modeled as the most stable (S)-1-indanol:DMSO complexes in a DMSO continuum yield satisfactory agreement with the experiment. Correspondence between experimental and simulated spectra is slightly improved when the VCD spectrum is calculated as the summed contributions of snapshots extracted from FPMD trajectories, due to better sampling of the potential-energy surface. Finally, NVPT calculations further improve the description of experimental spectra by taking into account higher-energy structures, which are not necessary local minima.
|


|
Pérez-Mellor, A., Alata, I., Lepere, V., & Zehnacker, A. (2018). Chirality effects in the structures of jet-cooled bichromophoric dipeptides. Journal of Molecular Spectroscopy, 349, 71–84.
Résumé: Diastereomer cyclic dipeptides built on a diketopiperazine (DKP) ring and phenylalanine residues of either identical or opposite chirality have been studied in jet-cooled conditions by combining conformer-specific IR-UV laser spectroscopy. Comparison between the IR-UV double resonance experiments and anharmonic calculations shows the presence of only one conformer of cyclo Phe-Phe, with one aromatic ring folded on the dipeptide DKP ring and the other one extended. This allows weak NH…π and CH…π interactions to take place, which are slightly different in cyclo SPhe-SPhe and cyclo SPhe-RPhe. In both diastereomers, comparison between the S0 and S1 spectra of the all 12C species and that containing one 13C indicates that the electronic excitation is localized on one aromatic ring. This study has been extended to linear SPhe-SPhe and linear SPhe-RPhe, already studied by Abo-Riziq (2005).
|


|
Peukert, S., Kijak, M., Ostapko, J., Sepiol, J., Le Bris, C., Zehnacker-Rentien, A., Gil, M., & Waluk, J. (2018). Supersonic jet spectroscopy of parent hemiporphycene: Structural assignment and vibrational analysis for S-0 and S-1 electronic states. Journal Of Chemical Physics, 149(13), 134307.

Résumé: Hemiporphycene (HPc), a constitutional isomer of porphyrin, is studied under supersonic expansion conditions by means of laser-induced fluorescence, visible-visible hole-burning experiments, single vibronic level fluorescence techniques, and quantum chemical calculations. Only one trans form of jet-cooled HPc is observed, in contrast to solution studies that evidence a mixture of two trans tautomeric forms separated in energy by similar to 1 kcal/mol. Reliable structural assignment is provided by simulating absorption and emission patterns at the density functional theory and time-dependent density functional theory levels of theory. The vibronic spectra are nicely reproduced for both electronic ground and lowest excited singlet states for the most stable trans form. In contrast to another porphyrin isomer, porphycene (Pc), no tunneling or photo-induced hydrogen transfer is detected. The lower symmetry of HPc compared with Pc and the concomitant non-equivalent positions of the inner-cavity nitrogen atoms result in a non-symmetric double minimum potential for tautomerization, larger energy barrier, and a longer tunneling distance, with the average intramolecular hydrogen bond length larger in HPc than in Pc. HPc readily forms hydrates that show red-shifted absorption relative to the bare molecule. Published by AIP Publishing.
|


|
Sekiguchi, T., Tamura, M., Oba, H., Carcarbal, P., Lozada-Garcia, R. R., Zehnacker-Rentien, A., Gregoire, G., Ishiuchi, S. - I., & Fujii, M. (2018). Molecular Recognition by a Short Partial Peptide of the Adrenergic Receptor: A Bottom-Up Approach. Angew Chem Int Ed Engl, 57, 5626–5629.
Résumé: Receptor-neurotransmitter molecular recognition is key for neurotransmission. Although crystal structures of the receptors are known, the mechanism for recognition is not clear. Reported here is the ultraviolet (UV) and infrared (IR) spectra of complexes between a partial peptide (SIVSF), mimicking the binding motif of a catechol ring in the adrenergic receptor, and various ligands. The UV spectra show that two isomers coexist in the complex of SIVSF with properly recognized ligands, such as protonated adrenaline (adrenalineH(+) ). From IR spectra, they are assigned to catechol- and amino-bound structures. The catechol-bound structure is not observed when the ligand is replaced by nonproper molecules, such as noradrenalineH(+) . The results suggest that SIVSF not only recognizes the catechol ring but can distinguish differences in the amine side chain. The method provides a new possibility for screening molecules as potential therapeutics for activating the receptor.
|


|
2017 |
Alata, I., Perez-Mellor, A., Ben Nasr, F., Scuderi, D., Steinmetz, V., Gobert, F., Jaidane, N. E., & Zehnacker-Rentien, A. (2017). Does the Residues Chirality Modify the Conformation of a Cyclo-Dipeptide? Vibrational Spectroscopy of Protonated Cyclo-diphenylalanine in the Gas Phase. J. Phys. Chem. A, 121(38), 7130–7138.

Résumé: The structure of a protonated diketopiperazine dipeptide, cyclo-diphenylalanine, is studied by means of infrared multiple photon dissociation spectroscopy combined with quantum chemical calculations. Protonation exclusively occurs on the oxygen site and, in the most stable conformer, results to an intramolecular OH center dot center dot center dot pi interaction, accompanied by a CH-pi interaction. Higher energy conformers with free OH and NH center dot center dot center dot pi interactions are observed as well, due to kinetic trapping. Optimization of the intramolecular interactions involving the aromatic ring dictates the geometry of the benzyl substituents. Changing the chirality of one of the residues has consequences on the CH center dot center dot center dot pi interaction, which is of C alpha H center dot center dot center dot pi nature for LD, while LL shows a C beta H center dot center dot center dot pi interaction. Higher-energy conformers also display some differences in the nature of the intramolecular interactions.
|


|
|
Bourguignon, B., Béroff, K., Bréchignac, P., Dujardin, G., Leach, S., & and Zehnacker-Rentien, A. (2017). In the wake of Physical Chemistry under irradiation: onward to the Institute of Molecular Sciences at Orsay. Histoire de la Recherche Contemporaine, 6, 16–27.
|


|
Perez-Mellor, A., & Zehnacker, A. (2017). Vibrational circular dichroism of a 2,5-diketopiperazine (DKP) peptide: Evidence for dimer formation in cyclo LL or LD diphenylalanine in the solid state. Chirality, 29(2), 89–96.
Résumé: The diastereomer diketopiperazine (DKP) peptides built on phenylalanine, namely, cyclo diphenylalanine LPhe-LPhe and LPhe-DPhe, were studied in the solid phase by vibrational circular dichroism (VCD) coupled to quantum chemical calculations. The unit structure of cyclo LPhe-LPhe in KBr pellets is a dimer bridged by two strong NH center dot center dot center dot O hydrogen bonds. The intense bisignate signature in the CO stretch region is interpreted in terms of two contributions arising from the free COs of the dimer and the antisymmetrical combination of the bound COs. In contrast, cyclo LPhe-DPhe shows no VCD signal in relation to its symmetric nature.
|


|
2016 |
Asselin, P., Madebene, B., Soulard, P., Georges, R., Goubet, M., Huet, T. R., Pirali, O., & Zehnacker-Rentien, A. (2016). Competition between inter- and intra-molecular hydrogen bonding: An infrared spectroscopic study of jet-cooled amino-ethanol and its dimer. Journal Of Chemical Physics, 145(22), 224313.

Résumé: The Fourier transform IR vibrational spectra of amino-ethanol (AE) and its dimer have been recorded at room temperature and under jet-cooled conditions over the far and mid infrared ranges (50-4000 cm(-1)) using the White-type cell and the supersonic jet of the Jet-AILES apparatus at the synchrotron facility SOLEIL. Assignment of the monomer experimental frequencies has been derived from anharmonic frequencies calculated at a hybrid CCSD(T)-F12/MP2 level. Various thermodynamical effects in the supersonic expansion conditions including molar dilution of AE and nature of carrier gas have been used to promote or not the formation of dimers. Four vibrational modes of the observed dimer have been unambiguously assigned using mode-specific scaling factors deduced from the ratio between experimental and computed frequencies for the monomer. The most stable g 'Gg' monomer undergoes strong deformation upon dimerization, leading to a homochiral head to head dimer involving two strong hydrogen bonds. Published by AIP Publishing.
|


|
Kumar, S., Lucas, B., Fayeton, J., Scuderi, D., Alata, I., Broquier, M., Le Barbu-Debus, K., Lepere, V., & Zehnacker, A. (2016). Photofragmentation mechanisms in protonated chiral cinchona alkaloids. Phys. Chem. Chem. Phys., 18(32), 22668–22677.
Résumé: The photo-stability of protonated cinchona alkaloids is studied in the gas phase by a multi-technique approach. A multi-coincidence technique is used to demonstrate that the dissociation is a direct process. Two dissociation channels are observed. They result from the C-8-C-9 cleavage, accompanied or not by hydrogen migration. The branching ratio between the two photo-fragments is different for the two pseudo-enantiomers quinine and quinidine. Mass spectrometry experiments coupling UV photo-dissociation of the reactants and structural characterization of the ionic photo-products by Infra-Red Multiple Photo-Dissociation (IRMPD) spectroscopy provide unambiguous information on their structure. In addition, quantum chemical calculations allow proposing a reactive scheme and discussing it in terms of the ground-state geometry of the reactant.
|


|
Lepere, V., Le Barbu-Debus, K., Clavaguera, C., Scuderi, D., Piani, G., Simon, A. L., Chirot, F., MacAleese, L., Dugourd, P., & Zehnacker, A. (2016). Chirality-dependent structuration of protonated or sodiated polyphenylalanines: IRMPD and ion mobility studies. Phys. Chem. Chem. Phys., 18(3), 1807–1817.
Résumé: Ion mobility experiments are combined with Infra-Red Multiple Photon Dissociation (IRMPD) spectroscopy and quantum chemical calculations for assessing the role of chirality in the structure of protonated and sodiated di- or tetra-peptides. Sodiated systems show a strong chirality dependence of the competition between Na+...O and Na+...pi interactions. Chirality effects are more subtle in protonated systems and manifest themselves by differences in the secondary interactions such hydrogen bonds between neutral groups or those involving the aromatic rings.
|


|
2015 |
Alata, I., Scuderi, D., Lepere, V., Steinmetz, V., Gobert, F., Thiao-Layel, L., Le Barbu-Debus, K., & Zehnacker-Rentien, A. (2015). Exotic Protonated Species Produced by UV-Induced Photofragmentation of a Protonated Dimer: Metastable Protonated Cinchonidine. Journal Of Physical Chemistry A, 119(39), 10007–10015.
Résumé: A metastable protonated cinchona alkaloid was produced in the gas phase by UV-induced photodissociation (UVPD) of its protonated dimer in a Paul ion trap. The infrared multiple photon dissociation (IRMPD) spectrum of the molecular ion formed by UVPD was obtained and compared to DFT calculations to characterize its structure. The protonation site obtained thereby is not accessible by classical protonation ways. The protonated monomer directly formed in the ESI source or by collision-induced dissociation (CID) of the dimer undergoes protonation at the most basic alkaloid nitrogen. In contrast, protonation occurs at the quinoline aromatic ring nitrogen in the UVPD-formed monomer.
|


|
Alauddin, M., Gloaguen, E., Brenner, V., Tardivel, B., Mons, M., Zehnacker-Rentien, A., Declerck, V., & Aitken, D. J. (2015). Intrinsic Folding Proclivities in Cyclic β-Peptide Building Blocks: Configuration and Heteroatom Effects Analyzed by Conformer-Selective Spectroscopy and Quantum Chemistry. Chem. Eur. J., 21(46), 16479–16493.

Résumé: This work describes the use of conformer-selective laser spectroscopy following supersonic expansion to probe the local folding proclivities of four-membered ring cyclic β-amino acid building blocks. Emphasis is placed on stereochemical effects as well as on the structural changes induced by the replacement of a carbon atom of the cycle by a nitrogen atom. The amide A IR spectra are obtained and interpreted with the help of quantum chemistry structure calculations. Results provide evidence that the building block with a trans-substituted cyclobutane ring has a predilection to form strong C8 hydrogen bonds. Nitrogen-atom substitution in the ring induces the formation of the hydrazino turn, with a related but distinct hydrogen-bonding network: the structure is best viewed as a bifurcated C8/C5 bond with the N heteroatom lone electron pair playing a significant acceptor role, which supports recent observations on the hydrazino turn structure in solution. Surprisingly, this study shows that the cis-substituted cyclobutane ring derivative also gives rise predominantly to a C8 hydrogen bond, although weaker than in the two former cases, a feature that is not often encountered for this building block.
|


|
Bouchet, A., Klyne, J., Piani, G., Dopfer, O., & Zehnacker, A. (2015). Diastereo-specific conformational properties of neutral, protonated and radical cation forms of (1R,2S)-cis- and (1R,2R)-trans-amino-indanol by gas phase spectroscopy. Physical Chemistry Chemical Physics, 17(39), 25809–25821.

Résumé: Chirality effects on the intramolecular interactions strongly depend on the charge and protonation states. Here, the influence of chirality on the structure of the neutral, protonated, and radical cation forms of (1R,2S)-cis- and (1R,2R)-trans-1-amino-2-indanol diastereomers, prototypical molecules with two chiral centers, is investigated in a molecular beam by laser spectroscopy coupled with quantum chemical calculations. The neutral systems are structurally characterised by double resonance IR-UV spectroscopy, while IR-induced dissociation spectroscopy is employed for the charged molecules. The sterical constraints due to the cyclic nature of the molecule emphasise the chirality effects, which manifest themselves by the formation of an intramolecular hydrogen bond in neutral or protonated (1R,2S)-cis-amino-indanol. In contrast, this interaction is not possible in (1R,2R)-trans-amino-indanol. In the protonated species, chirality also influences the spectroscopic probes in the NH/OH stretch range by fine-tuning subtle effects such as the hyperconjugation between the sigma(OH) orbital and sigma* orbitals localised on the alicyclic ring. The radical cation undergoes opening of the alicyclic ring, which results in an ionisation-induced loss of the chirality effects.
|


|
Mengesha, E. T., Zehnacker-Rentien, A., Sepiol, J., Kijak, M., & Waluk, J. (2015). Spectroscopic Study of Jet-Cooled Deuterated Porphycenes: Unusual Isotopic Effects on Proton Tunneling. J. Phys. Chem. B, 119(6), 2193–2203.

Résumé: Porphycene (Pc) is a well-known model for studying double hydrogen transfer, which shows vibrational-mode-specific tunneling splitting when isolated in supersonic jets or helium nanodroplets. The effect of deuteration on tunneling splitting is reported for jet-cooled heterogeneous, deuterated Pc samples (Pc-d(mix)) with the prevailing contribution of Pc-d(12) isotopologue. The sample introduced into the gas phase using laser desorption is studied by means of laser-induced fluorescence (LIF) and single vibronic level fluorescence (SVLF) measurements, in combination with quantum chemical calculations. The influence of molecular symmetry is studied by comparing Pc, Pc-d(12), and Pc-d(11). The spectra of Pc-d(12) show strong similarity to those of the parent undeuterated porphycene (Pc). Comparable tunneling splitting is observed in the two isotopologues, both for the 00 transition and the most efficient promoting 2A(g) mode. In contrast, an unusual isotopic effect is observed for the totally symmetrical 4A(g) mode. While this vibration behaves as a neutral mode in Pc, neither enhancing nor decreasing the tunneling efficiency, it strongly promotes hydrogen transfer in Pc-d(12). This observation is explained in terms of modification of the displacement vectors of the 4A(g) mode upon deuteration. It demonstrates that isotope substitution affects hydrogen transfer even when the weak structural modifications are far from the reaction center, emphasizing the strongly multidimensional nature of the tunneling process.
|


|
|
Zehnacker, A. (2015). Optical spectroscopy coupled with mass spectrometry methods. Physical Chemistry Chemical Physics, 17(39), 25672–25675.
|


|
2014 |
Bouchet, A., Altnoder, J., Broquier, M., & Zehnacker, A. (2014). IR-UV spectroscopy of jet-cooled 1-indanol: Restriction of the conformational space by hydration. JOURNAL OF MOLECULAR STRUCTURE, 1076, 344–351.
Résumé: The effect of hydration on a flexible amphiphilic molecule has been studied on the example of 1-hydroxyindan (1-indanol). Studies in jet-cooled conditions by means of resonance-enhanced two-photon ionization and IR-UV double resonance experiments show that the mono-hydrate 1-indanol(H2O) is formed in a dominant isomer, as well as the di-hydrate 1-indanol(H2O)(2). 1-Indanol(H2O) favors a cooperative hydrogen bond pattern with -OH center dot center dot center dot O(H)-H center dot center dot center dot pi it topology, while 1-indanol(H2O)(2) forms a cyclic hydrogen bond network with three OH center dot center dot center dot O interactions. The single conformation observed for the hydrates contrasts with the bare molecule which shows two dominant conformations, with the hydroxyl in axial or in equatorial position, respectively. Hydration therefore results in a restriction of the conformational space and conformational locking. (C) 2014 Elsevier B.V. All rights reserved.
|


|
Chaudret, R., de Courcy, B., Contreras-Garcia, J., Gloaguen, E., Zehnacker-Rentien, A., Mons, M., & Piquemal, J. P. (2014). Unraveling non-covalent interactions within flexible biomolecules: from electron density topology to gas phase spectroscopy. PHYSICAL CHEMISTRY CHEMICAL PHYSICS, 16(21), 9876–9891.

Résumé: The NCI (Non-Covalent Interactions) method, a recently-developed theoretical strategy to visualize weak non-covalent interactions from the topological analysis of the electron density and of its reduced gradient, is applied in the present paper to document intra- and inter-molecular interactions in flexible molecules and systems of biological interest in combination with IR spectroscopy. We first describe the conditions of application of the NCI method to the specific case of intramolecular interactions. Then we apply it to a series of stable conformations of isolated molecules as an interpretative technique to decipher the different physical interactions at play in these systems. Examples are chosen among neutral molecular systems exhibiting a large diversity of interactions, for which an extensive spectroscopic characterization under gas-phase isolation conditions has been obtained using state-of-the-art conformer-specific experimental techniques. The interactions presently documented range from weak intra-molecular H-bonds in simple amino-alcohols, to more complex patterns, with interactions of various strengths in model peptides, as well as in chiral bimolecular systems, where invaluable hints for the understanding of chiral recognition are revealed. We also provide a detailed technical appendix, which discusses the choices of cut-offs as well as the applicability of the NCI analysis to specific constrained systems, where local effects require attention. Finally, the NCI technique provides IR spectroscopists with an elegant visualization of the interactions that potentially impact their vibrational probes, namely the OH and NH stretching motions. This contribution illustrates the power and the conditions of use of the NCI technique, with the aim of providing an easy tool for all chemists, experimentalists and theoreticians, for the visualization and characterization of the interactions shaping complex molecular systems.
|


|
Gloaguen, E., Brenner, V., Alauddin, M., Tardivel, B., Mons, M., Zehnacker-Rentien, A., Declerck, V., & Aitken, D. J. (2014). Direct Spectroscopic Evidence of Hyperconjugation Unveils the Conformational Landscape of Hydrazides. ANGEWANDTE CHEMIE-INTERNATIONAL EDITION, 53(50), 13756–13759.
Résumé: The stereochemistry of hydrazides makes them especially interesting as building blocks for molecular design. An exhaustive conformational analysis of three model hydrazides was conducted in a conformer-selective approach by using a combination of high-level quantum chemistry calculations and vibrational spectroscopy in the gas phase and in solution. The NH stretch frequency was found to be highly sensitive to hyperconjugation, thus making it an efficient probe of the conformation of the neighboring nitrogen atom. This property greatly assisted the identification of the isomers observed experimentally in the conformer pool. A rationalization of the hydrazide conformational landscape is proposed, therefore paving the way for a better characterization of secondary structures in larger systems.
|


|
Scuderi, D., Lepere, V., Piani, G., Bouchet, A., & Zehnacker-Rentien, A. (2014). Structural Characterization of the UV-Induced Fragmentation Products in an Ion Trap by Infrared Multiple Photon Dissociation Spectroscopy. JOURNAL OF PHYSICAL CHEMISTRY LETTERS, 5(1), 56–61.
Résumé: Protonated cinchona alkaloids and their dimers undergo photochemical reaction in the gas phase, leading to UV-specific photofragments, not observed by collision-induced dissociation. Simultaneous coupling of UV and IR lasers with a Paul ion trap has been achieved for obtaining the vibrational spectrum of the fragments arising from the photodissociation. The structure of the photoproduced radical has been fully characterized by comparing the experimental spectrum to that simulated by DFT calculations.
|


|
Zehnacker, A. (2014). Chirality effects in gas-phase spectroscopy and photophysics of molecular and ionic complexes: contribution of low and room temperature studies. INTERNATIONAL REVIEWS IN PHYSICAL CHEMISTRY, 33(2), 151–207.
Résumé: This review focuses on chirality effects in spectroscopy and photophysics of chiral molecules or protonated ions, and their weakly bound complexes, isolated in the gas phase. Low-temperature studies in jet-cooled conditions allow disentangling the different interactions at play and shed light on the ancillary interactions responsible for chiral recognition, like OH...pi or CH...pi, which would be blurred at room temperature. The consequences of these interactions on chiral recognition in condensed phase are described, as well as the influence of higher energy conformers, which can be accessed in room-temperature experiments. The role of kinetic effects and solvation in jet-cooled experiments is discussed. Last, examples of dramatic chirality effects in photo-induced dissociation are given.
|


|
2013 |
|
Altnöder J., B. A., Lee J.J., Otto K.E., Suhm M.A., Zehnacker-Rentien A. (2013). Chirality-dependent balance between hydrogen bonding and London dispersion in isolated (±)-1-indanol clusters. PHYSICAL CHEMISTRY CHEMICAL PHYSICS, 15, 10167–10180.
|


|
Mahjoub, A., Le Barbu-Debus, K., & Zehnacker, A. (2013). Structural Rearrangement in the Formation of Jet-Cooled Complexes of Chiral (S)-1,2,3,4-Tetrahydro-3-isoquinolinemethanol with Methyl Lactate: Chirality Effect in Conformer Selection. JOURNAL OF PHYSICAL CHEMISTRY A, 117(14), 2952–60.

Résumé: The jet-cooled complexes between the two enantiomers of methyl lactate (ML) and (S)-1,2,3,4-tetrahydro-3-isoquinolinemethanol (THIQM) are studied by double resonance spectroscopy combined with ab initio calculations. Both diastereomer complexes exist in different isomers, involving either direct addition of THIQM on ML with no structural rearrangement of the subunits or formation of very stable structures involving multiple intermolecular hydrogen bonds and extensive deformation of the subunits. Competition between these two processes and its dependence upon chirality are discussed. It is shown that the most stable form of the chromophore (THIQMI with an OH···N hydrogen bond) prefers to directly stick to ML to form the addition complex whereas the second conformer (THIQMII with NH···O hydrogen bond) rearranges to form a strongly bound structure. The two structures are formed for the homochiral as well the heterochiral complex, however with different relative abundance. This shows an enantioselective binding preference of ML for one of the conformers of the chromophore.
|


|
Sen, A., Le Barbu-Debus, K., Scuderi, D., & Zehnacker-Rentien, A. (2013). Mass Spectrometry Study and Infrared Spectroscopy of the Complex Between Camphor and the Two Enantiomers of Protonated Alanine: The Role of Higher-Energy Conformers in the Enantioselectivity of the Dissociation Rate Constants. Chirality, 25, 436–443.

Résumé: The properties of the protonated complexes built from S camphor and R or S alanine were studied in a Paul ion trap at room temperature by collision-induced dissociation (CID) and infrared multiple-photon dissociation spectroscopy (IRMPD), as well as molecular dynamics and ab initio calculations. While the two diastereomer complexes display very similar vibrational spectra in the fingerprint region, in line with similar structures, and almost identical calculated binding energies, their collision-induced dissociation rates are different. Comparison of the IRMPD results to computed spectra shows that the SS and SR complexes both contain protonated alanine strongly hydrogen-bonded to the keto group of camphor. The floppiness of this structure around the NH+…O = C hydrogen bond results in a complex potential energy surface showing multiple minima. Calculating the dissociation rate constant within the frame of the transition state theory shows that the fragmentation rate larger for the heterochiral SR complex than the homochiral SS complex can be explained in terms of two almost isoenergetic low-energy conformers in the latter that are not present for the former. Chirality 00:000-000, 2013. © 2013 Wiley Periodicals, Inc.
|


|
|
Sen, A., Lepere, V., Le Barbu-Debus, K., & Zehnacker, A. (2013). How do Pseudoenantiomers Structurally Differ in the Gas Phase? An IR/UV Spectroscopy Study of Jet-Cooled Hydroquinine and Hydroquinidine. CHEMPHYSCHEM, 14(15), 3559–3568.
|


|
2012 |
Sen, A., Bouchet, A., Lepere, V., Le Barbu-Debus, K., Scuderi, D., Piuzzi, F., & Zehnacker-Rentien, A. (2012). Conformational Analysis of Quinine and Its Pseudo Enantiomer Quinidine: A Combined Jet-Cooled Spectroscopy and Vibrational Circular Dichroism Study. JOURNAL OF PHYSICAL CHEMISTRY A, 116(32), 8334–8344.

Résumé: Laser-desorbed quinine and quinidine have been studied in the gas phase by combining supersonic expansion with laser spectroscopy, namely, laser-induced fluorescence (LIF), resonance-enhanced multiphoton ionization (REMPI), and IR-UV double resonance experiments. Density functional theory (DFT) calculations have been done in conjunction with the experimental work. The first electronic transition of quinine and quinidine is of pi-pi* nature, and the studied molecules weakly fluoresce in the gas phase, in contrast to what was observed in solution (Qin, W. W.; et al. J. Phys. Chem. C 2009, 113, 11790). The two pseudo enantiomers quinine and quinidine show limited differences in the gas phase; their main conformation is of open type as it is in solution. However, vibrational circular dichroism (VCD) experiments in solution show that additional conformers exist in condensed phase for quinidine, which are not observed for quinine. This difference in behavior between the two pseudo enantiomers is discussed.
|


|
2011 |
Chakraborty, A., Guchhait, N., Le Barbu-Debus, K., Mahjoub, A., Lepere, V., & Zehnacker-Rentien, A. (2011). Role of Conformational Isomerism in Solvent-Mediated Charge Transfer in Chiral (S) 1,2,3,4-Tetrahydro-3-isoquinoline Methanol (THIQM): Condensed-Phase to Jet-Cooled Spectroscopic Studies. JOURNAL OF PHYSICAL CHEMISTRY A, 115(34), 9354–9364.

Résumé: Intramolecular charge-transfer reaction in chiral (S) 1,2,3,4-tetrahydro3-isoquinoline methanol (THIQM) has been investigated in the condensed phase and in jet-cooled conditions by means of laser-induced fluorescence, dispersed emission, resonance-enhanced two-photon ionization, and IR-UV double resonance experiments, as well as quantum chemical calculations. In the condensed phase, THIQM only shows local emission in nonpolar and protic solvents and dual emission in aprotic polar solvents, where the solvent-polarity dependent Stokes shifted emission is ascribed to a state involving charge transfer from the nitrogen lone pair to the benzene pi-cloud. Ab initio calculations reveal two low-energy conformers, which are observed in jet-cooled conditions. In the most stable conformer, THIQM(1), the CH(2)OH substituent acts as a hydrogen bond donor to the nitrogen lone pair in the equatorial position, while the second most stable conformer, THIQM(II), corresponds to the opposite NH center dot center dot center dot O hydrogen bond, with the nitrogen lone pair in the axial position. The two low-energy jet-cooled conformers of THIQM evidenced from the laser-induced fluorescence and dispersed emission spectra only show structured local emission. Complexes with usual solvents reproduce the condensed phase properties. The jet-cooled complex with aprotic polar, solvent acetonitrile shows both local emission and charge transfer emission as observed in solution. The jet-cooled hydrate mainly shows local emission due to the unavailability of the nitrogen lone pair through intermolecular hydrogen bonding.
|


|
Le Barbu-Debus, K., Sen, A., Broquier, M., & Zehnacker, A. (2011). Jet-cooled hydrates of Chiral (S) 1,2,3,4-tetrahydro-3-isoquinoline methanol (THIQM): structure and mechanism of formation. PHYSICAL CHEMISTRY CHEMICAL PHYSICS, 13(31), 13985–13991.
Résumé: The mechanism of formation of hydrates of chiral (S) 1,2,3,4-tetrahydro-3-isoquinoline (THIQM) with two water molecules has been investigated in jet-cooled condition by means of resonance-enhanced two-photon ionization and IR-UV double resonance experiments. Quantum chemical calculations reveal that only one isomer of the THIQM is involved in the THIQM-(H(2)O)(2) complex formation, in contrast with what was observed for THIQM-(H(2)O). Anharmonic vibration calculations allowed unambiguous assignment of THIQM-(H(2)O)(2) to a complex resulting from the addition of a water molecule on the most stable THIQM-(H(2)O) complex. A sequential mechanism for complex formation has been deduced from these results.
|


|
Scuderi, D., Le Barbu-Debus, K., & Zehnacker, A. (2011). The role of weak hydrogen bonds in chiral recognition. PHYSICAL CHEMISTRY CHEMICAL PHYSICS, 13(40), 17916–17929.

Résumé: Chiral recognition has been studied in neutral or ionic weakly bound complexes isolated in the gas phase by combining laser spectroscopy and quantum chemical calculations. Neutral complexes of the two enantiomers of lactic ester derivatives with chiral chromophores have been formed in a supersonic expansion. Their structure has been elucidated by means of IR-UV double resonance spectroscopy in the 3 mm region. In both systems described here, the main interaction ensuring the cohesion of the complex is a strong hydrogen bond between the chromophore and methyl-lactate. However, an additional hydrogen bond of much weaker strength plays a discriminative role between the two enantiomers. For example, the 1 : 1 heterochiral complex between R-(+)-2-naphthyl-ethanol and S-(+) methyl-lactate is observed, in contrast with the 1 : 1 homochiral complex which lacks this additional hydrogen bond. On the other hand, the same kind of insertion structures is formed for the complex between S-( +/-)-cis-1-amino-indan-2-ol and the two enantiomers of methyl-lactate, but an additional addition complex is formed for R-methyl-lactate only. This selectivity rests on the formation of a weak CH center dot center dot center dot pi interaction which is not possible for the other enantiomer. The protonated dimers of Cinchona alkaloids, namely quinine, quinidine, cinchonine and cinchonidine, have been isolated in an ion trap and studied by IRMPD spectroscopy in the region of the nu(OH) and nu( NH) stretch modes. The protonation site is located on the alkaloid nitrogen which acts as a strong hydrogen bond donor in all the dimers studied. While the nature of the intermolecular hydrogen bond is similar in the homochiral and heterochiral complexes, the heterochiral complex displays an additional weak CH center dot center dot center dot O hydrogen bond located on its neutral part, which results in slightly different spectroscopic fingerprints in the n( OH) stretch region. This first spectroscopic evidence of chiral recognition in protonated dimers opens the way to the study of the complexes of Cinchona alkaloids involved in enantioselective catalysis. These examples show how secondary hydrogen bonds controlled by stereochemical factors govern molecular recognition processes.
|


|
Sepiol, J., Grabowska, A., Borowicz, P., Kijak, M., Broquier, M., Jouvet, C., Dedonder-Lardeux, C., & Zehnacker-Rentien, A. (2011). Excited-state intramolecular proton transfer reaction modulated by low-frequency vibrations: An effect of an electron-donating substituent on the dually fluorescent bis-benzoxazole. JOURNAL OF CHEMICAL PHYSICS, 135(3), 034307.
Résumé: Excited-state intramolecular proton transfer (ESIPT) reaction has been studied in a molecule showing dual fluorescence, the 2,5-bis(2-benzoxazolyl)-4-methoxyphenol (BBMP), and its isotopomers, where the methoxy, and alternatively, the OH group has been deuterated. Attention is focused on the influence of electron donating OCH3 substituent on fast excited state reaction. Comparison between the resonance-enhanced multiphoton ionization spectrum and the laser-induced excitation of the primary and phototautomeric emissions has been done. The geometry, electron density distribution, vibrational structure as well as the potential energy profiles in the S-0 and S-1 states of four possible rotameric forms of BBMP were calculated with application of the density functional theory (DFT). It allowed identifying the most probable conformer and assessing the role of low-frequency motions for the ESIPT efficiency. (C) 2011 American Institute of Physics. [doi:10.1063/1.3609759]
|


|
2010 |
Albrecht, M., Borba, A., Le Barbu-Debus, K., Dittrich, B., Fausto, R., Grimme, S., Mahjoub, A., Nedic, M., Schmitt, U., Schrader, L., Suhm, M. A., Zehnacker-Rentien, A., & Zischang, J. (2010). Chirality influence on the aggregation of methyl mandelate. NEW JOURNAL OF CHEMISTRY, 34(7), 1266–1285.

Résumé: The methyl ester of mandelic acid is investigated by a wide range of techniques to unravel its aggregation pattern and the influence of relative chirality of the aggregating monomers. Matrix isolation confirms that a single monomer conformation prevails. The electronic spectrum of the dimers is strongly affected by the relative monomer chirality. Vibrational effects are more subtle and can be explained in terms of the most stable homo- and heteroconfigurational dimer structures, when compared to results of MP2 and DFT-D computations. Selective IR/UV double resonance techniques and wide-band FTIR spectroscopy provide largely consistent spectroscopic fingerprints of the chirality discrimination phenomena. The dominant homochiral dimer has two intermolecular O-H center dot center dot center dot O=C hydrogen bonds whereas the more strongly bound heterochiral dimer involves only one such hydrogen bond. This is a consequence of the competition between dispersion and intramolecular or intermolecular hydrogen bonding. Aromatic interactions also play a role in trimers and larger clusters, favoring homochiral ring arrangements. Analogies and differences to the well-investigated methyl lactate system are highlighted. Bulk phases show a competition between different hydrogen bond patterns. The enantiopure, racemic, and 3 : 1 crystals involve infinite hydrogen-bonded chains with different arrangements of the aromatic groups. They exhibit significantly different volatility, the enantiopure compound being more volatile than the racemic crystal. The accumulated experimental and quantum-chemical evidence turns methyl mandelate into a model system for the role of competition between dispersion forces and hydrogen bond interactions in chirality discrimination.
|


|
|
Anne Zehnacker Ed. (2010). Chiral Recognition in the Gas Phase. CRC Press Taylor & Francis Group, .
|


|
Chakraborty, A., Le Barbu-Debus, K., Zehnacker-Rentien, A., & Guchhait, N. (2010). Laser-induced fluorescence and dispersed fluorescence studies of the donor-acceptor system 4-amino 3-methyl benzoic acid methyl ester and its solvated clusters: Evidence of excited-state charge-transfer reaction. JOURNAL OF PHOTOCHEMISTRY AND PHOTOBIOLOGY A-CHEMISTRY, 213(2-3), 164–170.
Résumé: Laser-induced fluorescence (LIF) excitation and dispersed fluorescence (OF) spectra of 4-amino 3-methyl benzoic acid methyl ester (AMBME) and its solvated clusters with solvents such as methanol, water and acetonitrile have been investigated in a supersonic expansion. Spectral signature supports the presence of two conformers in the cooled jet which is in line with theoretical calculations. In addition to structured local emission from the Franck Condon excited state, the molecule AMBME shows red-shifted broad emission from the state attributed to the close-lying charge transfer (CT) state, which is facilitated by exciting low-frequency modes. The molecule readily forms clusters with different solvents and the clusters' electronic excitation bands appear in the low-energy side of the transition origin of the bare molecule. Excitation of the clusters leads to the appearance of red-shifted solvent-polarity dependent CT emission. (C) 2010 Elsevier B.V. All rights reserved.
|


|
Scuderi, D., Maitre, P., Rondino, F., Le Barbu-Debus, K., Lepere, V., & Zehnacker-Rentien, A. (2010). Chiral Recognition in Cinchona Alkaloid Protonated Dimers: Mass Spectrometry and UV Photodissociation Studies. JOURNAL OF PHYSICAL CHEMISTRY A, 114(9), 3306–3312.

Résumé: Chiral recognition in protonated cinchona alkaloid dimers has been studied in mass spectrometry experiments. The experimental setups involved a modified 7T FT-ICR (Fourier transform-ion cyclotron resonance) mass spectrometer (MS) and a modified Paul ion trap both equipped with an electrospray ionization source (ESI). The Paul ion trap has been coupled to a frequency-doubled dye laser. The fragmentation of protonated dimers made from cinchonidine (Cd) and the two pseudoenantiomers of quinine, namely, quinine (Qn) and quinidine (Qd), has been assessed by means of collision-induced dissociation (CID) as well as UV photodissociation (UVPD). Whereas CID fragmentation of the dimers only leads to the evaporation of the monomers, UVPD results in the additional loss of a neutral radical fragment corresponding to the quinuclidinyl radical. The effect of the excitation wavelength and of complexation with H(2)SO(4) has been studied to cast light on the reaction mechanism. Complexation with H(2)SO(4) modifies the photoreactivity of the dimers; only evaporation of the monomeric fragments, quinine, and cinchonidine is observed. Comparison between the mass spectra of the cinchona alkaloid (CdQnH(+)) or (CdQdH(+)) dimers resulting from the UVPD of (CdQnH(2)SO(4)H(+)) and that of bare (CdQnH(+)) helps propose a fragmentation mechanism, which is thought to involve fast proton transfer from the quinuclidine part of a molecular subunit to the quinoline ring. CID and UV fragmentation experiments show that the homochiral dimer is more strongly bound than the heterochiral adduct.
|


|
2009 |
Le Barbu-Debus, K., Broquier, M., Mahjoub, A., & Zehnacker-Rentien, A. (2009). Chiral recognition in jet-cooled complexes of (1R,2S)-(+)-cis-1-amino-2-indanol and methyl lactate: on the importance of the CH...pi interaction. PHYSICAL CHEMISTRY CHEMICAL PHYSICS, 11(35), 7589–7598.

Résumé: Complexation between (1R,2S)-(+)-cis-1-amino-2-indanol (AI) and the two enantiomers of methyl lactate has been studied by means of laser-induced fluorescence, resonance-enhanced two-photon ionisation, and IR-UV double resonance spectroscopy, in the region of 3 μm. Two isomeric complexes have been spectroscopically characterised for each diastereoisomer. Comparison with ab initio calculations shows that the most stable form is an insertion structure, common to the two diastereoisomers, in which the OH group of methyl lactate inserts into the intramolecular bond of AI. This structure shows almost no chiral discrimination. A secondary structure has been observed, which is specific to each enantiomer. It involves a main hydrogen bond from the OH group of methyl lactate to AI together with weaker hydrogen bonds, which depend on chirality. The enantioselectivity in the hydrogen bond topology is due to a weak stabilizing CH center dot center dot center dot pi interaction, involving the CH located on the asymmetric carbon of methyl lactate, which can be obtained for one of the enantiomers only.
|


|
Mahjoub, A., Chakraborty, A., Lepere, V., Le Barbu-Debus, K., Guchhait, N., & Zehnacker, A. (2009). Chirality-dependent hydrogen bond direction in jet-cooled (S)-1,2,3,4-tetrahydro-3-isoquinoline methanol (THIQM): IR-ion dip vibrational spectroscopy of the neutral and the ion. PHYSICAL CHEMISTRY CHEMICAL PHYSICS, 11(25), 5160–5169.

Résumé: The structural modifications of (S)-1,2,3,4-tetrahydro-3-isoquinoline methanol (THIQM) upon ionisation have been investigated in jet-cooled conditions, by means of laser-induced fluorescence, REMPI, and IR-UV ion-dip spectroscopy of the neutral ground state and the ion. These results combined with ab initio calculations, support the presence in the jet of two low-energy conformers of THIQM. In the most stable Conformer I, the CH(2)OH substituent acts as a hydrogen bond donor to the nitrogen lone pair in the equatorial position. In this case, the nitrogen atom is in ( S) configuration. Conformer II shows the opposite NH center dot center dot center dot O hydrogen bond from the hydrogen atom in the equatorial position of nitrogen to the OH group. In this case, the nitrogen atom is in ( R) configuration. This chirality dependence of the hydrogen bond direction is lost upon ionisation. While ionisation of Conformer II reinforces the NH center dot center dot center dot O hydrogen bond, ionisation of Conformer I induces its isomerisation to the same ion as Conformer II, i.e. a change in hydrogen bond direction.
|


|
2008 |
Le Barbu-Debus, K., Broquier, M., Mahjoub, A., & Zehnacker-Rentien, A. (2008). Chiral recognition between alpha-hydroxylesters: A double-resonance IR/UV study of the complexes of methyl mandelate with methyl glycolate and methyl lactate. JOURNAL OF PHYSICAL CHEMISTRY A, 112(40), 9731–9741.

Résumé: Chiral recognition between alpha hydroxylesters has been studied in jet-cooled complexes of methyl mandelate with methyl lactate. The complex with nonchiral methyl glycolate has also been studied for the sake of comparison. The hydrogen-bond topology of the complexes has been interrogated by means of IR/UV double-resonance spectroscopy in the range of 3 μm. A theoretical approach has been conducted in conjunction with the experimental work to assist in the analysis of the spectra. Owing to the conformational flexibility of the subunits at play, emphasis has been put on the methodology used for the exploration of the potential-energy surface. The hydrogen-bond topology is very similar in the homo- and heterochiral complexes. It involves insertion of the hydroxyl group of methyl mandelate within the intramolecular hydrogen bond of methyl lactate or methyl glycolate, resulting in a five-membered ring. This contrasts with methyl lactate clusters previously studied by FTIR spectroscopy in a filet jet.(1).
|


|
Zehnacker, A., & Suhm, M. A. (2008). Chirality recognition between neutral molecules in the gas phase. ANGEWANDTE CHEMIE-INTERNATIONAL EDITION, 47(37), 6970–6992.

Résumé: Noncovalent interactions are particularly intriguing when they involve chiral molecules, because the interactions change in a subtle way upon replacing one of the partners by its mirror image. The resulting phenomena involving chirality recognition are relevant in the biosphere, in organic synthesis, and in polymer design. They may be classified according to the permanent or transient chirality of the interacting partners, leading to chirality discrimination, chirality induction, and chirality synchronization processes. For small molecules, high-level quantum chemical calculations for such processes are feasible. To provide reliable connections between theory and experiment, such phenomena are best studied in vacuum isolation at low temperature, using rotational, vibrational, electronic, and photoionization spectroscopy. We review these techniques and the results which have become available in recent years, with special emphasis on dimers of permanently chiral molecules and on the influence of conformational flexibility. Analogies between the microscopic mechanisms and macroscopic phenomena and between intra- and intermolecular cases are drawn.
|


|
2007 |
|
Deperasinska, I., Zehnacker, A., Lahmani, F., Borowicz, P., & Sepiol, J. (2007). Fluorescence studies of terrylene in a supersonic jet: Indication of a dark electronic state below the allowed transition. JOURNAL OF PHYSICAL CHEMISTRY A, 111(20), 4252–4258.
|


|
|
Le Barbu-Debus, K., Guchhait, N., & Zehnacker-Rentien, A. (2007). Electronic and infrared spectroscopy of jet-cooled (+/-)-cis-1-amino-indan-2-ol hydrates. PHYSICAL CHEMISTRY CHEMICAL PHYSICS, 9(32), 4465–4471.
|


|
|
Mahjoub, A., Le Barbu-Debus, K., & Zehnacker-Rentien, A. (2007). Double resonance IR/UV study of the complexes of methyl mandelate with ethyl glycolate and methyl lactate. In Fundamental and Applied Spectroscopy (Vol. 935, pp. 208–213).
|


|

 Spectroscopie électronique et vibrationnelle en phase gazeuse
Spectroscopie électronique et vibrationnelle en phase gazeuse
 Liaison hydrogène
Liaison hydrogène
 Transfert d’électron et de proton photo-induits
Transfert d’électron et de proton photo-induits
 Reconnaissance moléculaire et reconnaissance chirale
Reconnaissance moléculaire et reconnaissance chirale
 Dichroïsme circulaire vibrationnel
Dichroïsme circulaire vibrationnel
 La translocation du proton par effet tunnel dans des dérivés du porphycène
La translocation du proton par effet tunnel dans des dérivés du porphycène
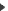 les effets d’hyperconjugaison dans des hydrazides en collaboration avec Valérie Declerck et David Aitken de l’ICCMO/Orsay et Michel Mons, Eric Gloaguen, Valérie Brenner du Lidyl/CEN Sacaly
les effets d’hyperconjugaison dans des hydrazides en collaboration avec Valérie Declerck et David Aitken de l’ICCMO/Orsay et Michel Mons, Eric Gloaguen, Valérie Brenner du Lidyl/CEN Sacaly Comparison of stereochemical effects on the structure of a neutral, radical cation and protonated molecule en collaboration avec Aude Bouchet et Otto Dopfer Université Technique de Berlin
Comparison of stereochemical effects on the structure of a neutral, radical cation and protonated molecule en collaboration avec Aude Bouchet et Otto Dopfer Université Technique de Berlin

