Home > Research Teams > Structure and Dynamics of Isolated Complex and Photoexcited Systems > MOlecules in MAtrices > Last publications > The keto/gem-diol hydration equilibrium
The keto/gem-diol hydration equilibrium
by - 25 March 2021 (modifié le 29 April 2021)
The keto/gem-diol hydration reaction explored by an ab-initio molecular dynamics study.
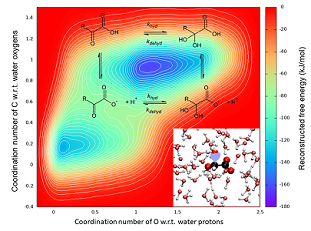
Reversible Hydration of α‑Dicarbonyl Compounds from Ab Initio Metadynamics Simulations: Comparison between Pyruvic and Glyoxylic Acids in Aqueous Solutions.
R. Pollet and W. Chin - J. Phys. Chem. B (2021), 125, 11, 2942–2951
Atmospheric aerosols have a strong impact on the climate and on human health: they can absorb or reflect solar radiations; they play an important role in cloud formation; they contribute to the formation of particle matter which badly affect human health. In particular, glyoxylic acid and pyruvic acid are highly abundant in aerosols, especially in marine aerosols. In the aqueous phase, glyoxylic and pyruvic acids adopt two forms in equilibrium: The keto form with two carbonyl groups and the gem-diol form that bears two hydroxyl groups on the same carbon. While the gem-diol form is transparent to solar radiation, the keto moiety is light sensitive, thereby contributing through its photochemistry to the production of larger compounds involved in the formation of secondary organic aerosols.
By means of ab-initio simulations well suited to the observation of rare events, our theoretical study on the keto/gem-diol equilibrium sheds light on the hydration mechanism of glyoxylic and pyruvic acids. Our investigation also provides a detailed description of the solvation sites and of their conformational landscape in solution.