Home > Headlines > Understanding the coupling of the hydrated proton to its first solvation shell
Understanding the coupling of the hydrated proton to its first solvation shell
by - 23 October 2022
Despite its considerable importance, the "Grotthus" mechanism; by which an ’excess’ proton or proton defect diffuses through the hydrogen bond network of water molecules in aqueous solution; remains largely misunderstood. In liquid water, the solvation of these excess protons is represented in two main forms: H9O4+ (Eigen cation) and H5O2+ (Zundel cation). Very recently, experimental works concluded that the Zundel cation would be the predominant form, but even more recent simulations seem to indicate that it is, on the contrary, the Eigen cation which dominates.
Full-dimensional quantum simulations (up to 33 dimensions) based on the Multi-Layer Multi-Configuration Time-Dependent Hartree (ML-MCTDH) method have reproduced the Infra-red spectra of the Zundel and Eigen cations. These calculations have allowed a detailed understanding of the couplings between the proton and the water molecules that surround it in its first solvation shell.
The conclusion is that the H5O2+ subunit is the fundamental subunit that explains the two spectra. Embedded in the static environment of the parent Eigen cation, this subunit reproduces the positions and broadenings of its main excess-proton bands. In isolation, its spectrum reverts to the well-known Zundel ion. Hence, the dynamics of this subunit polarized by an environment suffice to explain the spectral signatures and anharmonic couplings of the solvated proton in its first solvation shell
The new results do not bring information about the relative populations of the two structures, but stress that the difficulty to understand if one structure dominates may partly come from the fact that the H5O2+ subunit can exhibit very similar spectral signatures compared to the Eigen cation when placed in a polarizing environment.
This finding, backed by the full quantum-mechanical approach, is suggestive of picturing the Eigen cation as three symmetric overlapping and strongly polarized Zundel H5O2+ subunits in the spirit of the classical ‘special pair dance’ models of the solvated proton.
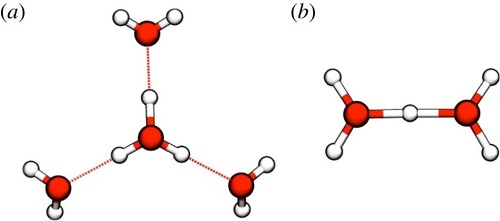
- Fig. 1
- Eigen cation, H9O4+ (a) ; Zundel cation, H5O2+ (b).
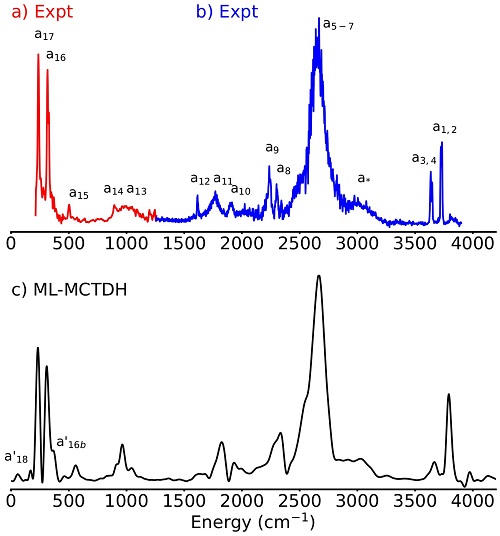
- Fig. 2
- Experimental and theoretical spectra of the Eigen cation
Contact: Fabien Gatti
Schröder, M., Gatti, F., Lauvergnat, D. et al. The coupling of the hydrated proton to its first solvation shell.
Nat Commun 13, 6170 (2022)
Also in this section :
- Webb Unveils the Dark Side of Pre-stellar Ice Chemistry
- Attosecond science to define the infinitely short
- Understanding the processes induced by electrons
- Distant formation and early evolution of the carbonaceous asteroid Ryugu: direct evidence from samples returned by Hayabusa2
- CinNapht : a new fluorophore family
- In search of hidden order
- Death of Roland Lefebvre
- Manipulating electrons with light
- The Etoiles de l’Europe’s special prize has been awarded to Ruxandra Gref
- Death of Michel Barat
- First Bose-Einstein condensation in space


