Stage de niveau M2 dans l'équipe DIRAM
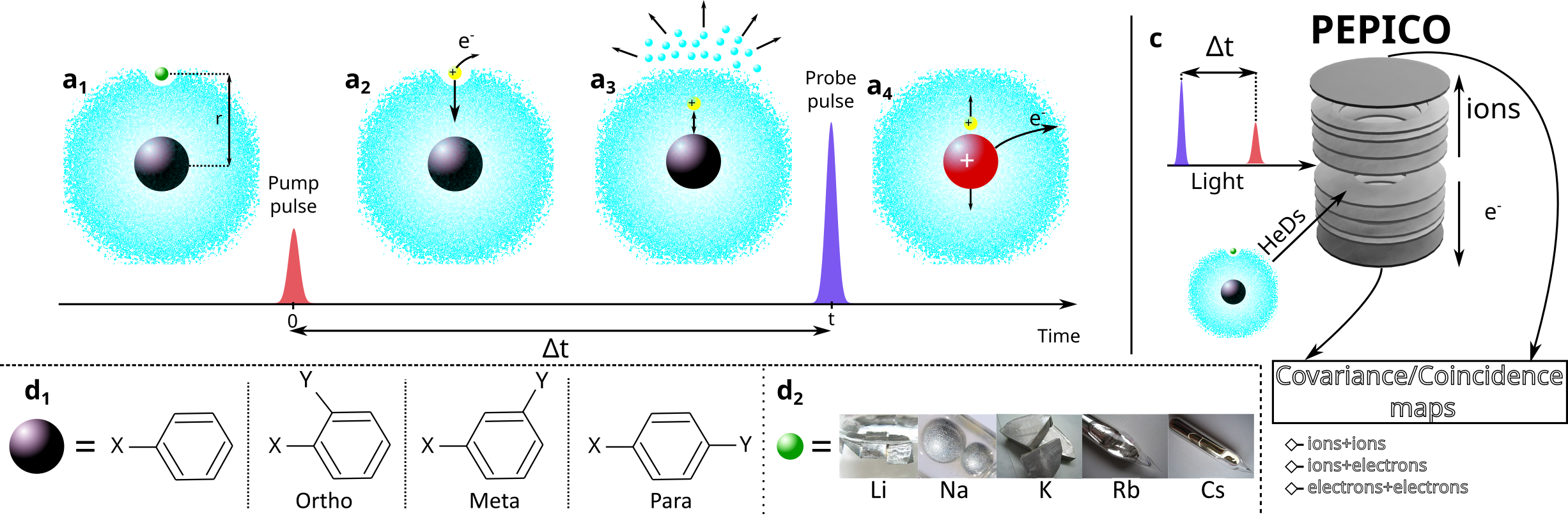
Figure 1: Proposed experimental scheme. (a1) A HeD is doped with an alkali atom (green sphere) and a molecule (black sphere) initially separated by a distance r. (a2) A laser pulse ionizes the alkali atom triggering the start of the reaction (a3) the two species meet and stabilize by evaporation of helium atoms (a4) After a delay Δt, a second laser pulse ionizes the formed complex inducing a Coulomb explosion. (d1) List of substitution patterns on the benzene ring considered for the project. X and Y refer to substituents of the hydrogen atoms that will modify the dipole moment and the polarizability of the system according to their nature and relative position. (d2) Relevant alkali atoms for the project with their electronic configuration
Ultrashort laser pulses have revolutionized our ability to track molecular rearrangements in real time with femtosecond resolution (1 fs = 10-15s), opening new frontiers in understanding chemical reaction dynamics. Generating and controlling these processes, on such rapid timescales, open up exciting opportunities for innovative applications in both chemistry and in biology, which could significantly impact society.
In this context, this project directly addresses the problematic of observing chemical bond formation in real time. This experimental internship at ISMO (Institut des Sciences Moléculaires d’Orsay) aims to tackle it by combining an ultracold (0.37K) superfluid solvent, hereby named Helium droplets1 (HeDs), and ultrashort laser pulses in the femtosecond range with tunable excitation radiation (240-800nm).
We will rely on time-resolved photoionization spectroscopy2, a technique based on the ionization of the species by the laser pulses and on the detection of the electrons and ions as molecular probes. The electron carries information about the initial electronic state of the ionized species, while the ion reveals the final state after relaxation processes such as fragmentation or isomerization.
In particular, we will use a pump-probe scheme (Fig. 1) to follow in real time the diving of an alkali ion (Fig. 1 d2) inside the HeD3 and its reaction with benzene-like molecules. This class of molecules allows to play with electrostatic properties (Fig. 1 d1), such as the dipole and quadrupole, by changing the group attached to it. We will investigate the evolution of the bond formation dynamics and the structure of the formed ionic complex according to the nature and the position of the group. We will compare the experimental results with high-level ab-initio theory and aim at developing phenomenological models to unravel the role of the solvent in the reaction.
Application Requirements
We seek candidates at the Master’s level in physics, chemistry, or related fields with a strong interest in experimental physics and instrumentation. Programming experience, particularly in Python, is beneficial, along with motivation for fundamental research and technical problem-solving.
Expected Outcomes
You will contribute to high-impact publications in ultrafast science and cold molecules while developing highly sought-after skills for both academic and industrial careers. This position represents a unique opportunity to develop cutting-edge instrumentation while contributing to fundamental advances in chemical reaction dynamics, combining advanced technology development with fundamental science.
[1] J. P. Toennies et A. F. Vilesov, Superfluid Helium Droplets: A Uniquely Cold Nanomatrix for Molecules and Molecular Complexes Angew. Chem. Int. Ed. 43 (2004), doi: 10.1002/anie.200300611.
[2] M. S. Schuurman et V. Blanchet, Time-resolved photoelectron spectroscopy: the continuing evolution of a mature technique Phys. Chem. Chem. Phys. 24 (2022), doi: 10.1039/D1CP05885A.
[3] S. H. Albrechtsen, C. A. Schouder, A. Viñas Muñoz, J. K. Christensen, C. Engelbrecht Petersen, M. Pi, M. Barranco, et H. Stapelfeldt, Observing the primary steps of ion solvation in helium droplets Nature 623 (2023), doi: 10.1038/s41586-023-06593-5.
For more information please contact:
Constant Schouder
email:
tel: 01 69 15 75 51